Журнал «Медицина неотложных состояний» №1(96), 2019
Вернуться к номеру
Особливості ліпідного профілю та кардіогемодинаміки при хронічному обструктивному захворюванні легень та коморбідності
Авторы: T.S. Ospanova, Zh.D. Semydotska, I.O. Chernyakova, O.V. Avdeyeva, O.M. Pionova, N.S. Tryfonova
Kharkiv National Medical University, Kharkiv, Ukraine
Рубрики: Медицина неотложных состояний
Разделы: Клинические исследования
Версия для печати
Актуальність. Хронічне обструктивне захворювання легень є класичним прикладом коморбідного стану, що характеризується поєднанням ішемічної хвороби серця, артеріальної гіпертензії та метаболічного синдрому. Метою дослідження було вивчення особливостей взаємозв’язку між параметрами ліпідного спектра та ехокардіографії в пацієнтів iз хронічним обструктивним захворюванням легень, ішемічною хворобою серця та артеріальною гіпертензією. Матеріали та методи. Обстежено 35 пацієнтів iз хронічним обструктивним захворюванням легень і коморбідною патологією (57,14 % чоловіків, 42,86 % жінок). Середній вік становив 57,0 рокiв, середня тривалість захворювання — 10,02 [5,0–15,0] року. У 23 пацієнтiв виявлено коморбідність з ішемічною хворобою серця та артеріальною гіпертензією, 11 осіб мали цукровий діабет 2-го типу, 8 — ішемічну хворобу серця (кардіосклероз, стенокардія), 4 — артеріальну гіпертензію. Результати. Пацієнти були розділені на 3 групи: В (n = 12), С (n = 14), D (n = 9). У групі B виявлено 3 позитивні вірогідні кореляції діаметра аорти з рiвнями загального холестерину, ліпопротеїнiв низької та дуже низької щільності. У групі С є 2 кореляції з тиском у легеневій артерії: позитивна — вмiсту ліпопротеїнiв низької щільності та негативна — ліпопротеїнiв високої щільності, а також негативний зв’язок між рiвнем тригліцеридiв та діастолічним об’ємом лівого шлуночка. У групі D кількість кореляцій збільшується до 6: з’являються кореляції вмiста ліпопротеїнів низької щільності з параметрами правого передсердя та шлуночка, а також лівого шлуночка. Було зазначено, що кількість кореляцій зростає удвічі за умови коморбідності з ішемічною хворобою серця. Висновки. Прогресування хронічного обструктивного захворювання легень та його поєднання iз супутнiми станами приводить до збільшення кількості кореляційних зв’язків між ліпідним спектром та показниками ехокардіографії, особливо при коморбідності з ішемічною хворобою серця.
Актуальность. Хроническая обструктивная болезнь легких является классическим примером коморбидного состояния, характеризующегося сочетанием ишемической болезни сердца, артериальной гипертензии и метаболического синдрома. Целью исследования было изучение особенностей взаимосвязи между параметрами липидного спектра и эхокардиографии у пациентов с хронической обструктивной болезнью легких, ишемической болезнью сердца и артериальной гипертензией. Материалы и методы. Обследовано 35 пациентов с хронической обструктивной болезнью легких и сопутствующей патологией (57,14 % мужчин, 42,86 % женщин). Средний возраст составил 57,0 года, средняя продолжительность заболевания — 10,02 [5,0–15,0] года. У 23 пациентов выявлена коморбидность с ишемической болезнью сердца и артериальной гипертензией, у 11 пациентов был сахарный диабет 2-го типа, у 8 — ишемическая болезнь сердца (кардиосклероз, стенокардия), у 4 — артериальная гипертензия. Результаты. Пациенты были разделены на 3 группы: B (n = 12), C (n = 14), D (n = 9). В группе B выявлены 3 положительные достоверные корреляции диаметра аорты с уровнями общего холестерина, липопротеинов низкой и очень низкой плотности. В группе С имеются 2 корреляции с давлением в легочной артерии: положительная — содержания липопротеинов низкой плотности и отрицательная — липопротеинов высокой плотности, а также отрицательная связь между уровнем триглицеридов и диастолическим объемом левого желудочка. В группе D число корреляций увеличивается до 6: появляются корреляции содержания липопротеинов низкой плотности с параметрами правого предсердия и желудочка, а также левого желудочка. Было отмечено, что при коморбидности с ишемической болезнью сердца количество корреляций увеличивается в два раза. Выводы. Прогрессирование хронической обструктивной болезни легких и ее сочетание с сопутствующими состояниями приводит к увеличению количества корреляционных связей между липидным спектром и показателями эхокардиографии, особенно при коморбидности с ишемической болезнью сердца.
Background. Chronic obstructive pulmonary disease is a classic example of a comorbid condition characterized by a combination with coronary artery disease, hypertension, and metabolic syndrome. The purpose of the study was to investigate the features of the correlation between the lipid spectrum and echocardiography parameters in patients with chronic obstructive pulmonary disease, coronary artery disease and hypertension. Materials and methods. Thirty-five patients with chronic obstructive pulmonary disease and comorbidity (57.14 % of males, 42.86 % of females) were examined. The average age was 57.0 years, the mean duration of the disease — 10.02 [5.0–15.0] years. Twenty three patients had comorbidity with coronary artery disease and hypertension, 11 persons had type 2 diabetes mellitus, 8 patients — coronary artery disease (cardiosclerosis, angina pectoris), 4 individuals — stage 2 hypertension. Results. Patients were divided into 3 groups: B (n = 12), C (n = 14), D (n = 9). In group B, 3 positive significant correlations of aortic diameter with total cholesterol, low and very low density lipoproteins were revealed. In group C, there are 2 correlations with systolic pressure in pulmonary artery: positive — of low density lipoproteins and negative — of high-density lipoproteins, and negative association between triglycerides and left ventricular diastolic volume. In group D, the number of correlations increases to 6: correlations of low density lipoproteins with parameters of right atrium and right ventricle appear, as well as left ventricle. It was noted that an increase in the severity of chronic obstructive pulmonary disease and comorbidity with hypertension does not lead to increased number of correlations, but coronary artery disease increases the number of correlations 2-fold. Conclusions. As the severity of the chronic obstructive pulmonary disease progresses, the number of correlations between the lipid spectrum and structural and functional state of the myocardium increases according to the echocardiography data.
коморбідність; хронічне обструктивне захворювання легень; ехокардіографія
коморбидность; хроническая обструктивная болезнь легких; эхокардиография
comorbidity; chronic obstructive pulmonary disease; echocardiography
The mind that opens to a new idea
never returns to its original size.
Albert Einstein
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the current problems of contemporary medicine due to the rapid increase in morbidity, disability and mortality rates (more than 30 % in 10 years), high treatment costs and decline in the patient’s quality of life [1–5]. COPD will have taken the third place among the mortality causes by 2030 according to the World Health Organization [2, 6].
At the end of the 20th century, the number of patients with persistent combinations of various diseases had increased rapidly. Those combinations were called comorbidities [7, 8]. Comorbidity has become the main informational source for studying the general mechanisms of various diseases [9].
The prevalence of concomitant diseases in patients with COPD is higher than in the whole population [10–12]. It attests that COPD is a classic example of a comorbid condition, which is characterized by a combination with coronary artery disease (CAD), arterial hypertension (AH), and metabolic syndrome.
In recent years, there has been a growing interest in studies of structural and functional disorders of the myocardium using echocardiography in COPD with AH and CAD comorbidity [3, 13–16]. The possibility of early diagnosis of heart failure, diastolic dysfunction of both ventricles by means of echocardiography is noted [17, 18]. However, a consensus about the nature of right and left ventricular dysfunction and its relationship with COPD is not found [19].
The results of studying the structural and functional parameters of the myocardium in COPD using echocardiography indicate the effect of pulmonary hypertension not only on the right ventricle (RV), but also on the left ventricle (LV). It is so-called interventricular interaction, which is caused by the anatomical and electrophysiological connections of both ventricles.
Cardiac remodeling in COPD and cardiovascular comorbidity (CAD and AH) is recognized as a universal process based on chronic systemic inflammation [11, 12, 20]. COPD is one of the most frequent causes of both ventricles diastolic dysfunction [15].
The achievements of modern network science in the field of molecular biology, genome and molecular genetics made it possible to study the causes of comorbidity at the body, cellular, molecular, genetic levels. Also, they enable to show the importance of cell signaling between these components and to allocate a new discipline, network medicine, representing a perspective approach to understanding diseases from a network point of view, reclassification of complicated syndromological diseases [21–24]. These achievements allow selecting a special group of syntropic diseases with similar etiological and pathogenetic mechanisms. The similarity between these diseases is determined by the general predisposition genes. The modification of universal network processes at the genomic, molecular and cellular levels leads to the development of syntropic diseases. They manifest at the organ and body levels in systemic chronic inflammation of target organs. This creates new properties of various systems (emergence), which are difficult to explain using only the signs of individual diseases and their components.
It is believed that network approaches will be used to integrate several genotypes to investigate the pathogenesis of the disease and to resolve the problem of synthropy and comorbidity in further studies of COPD genetics.
All of the above emphasizes the urgency of studying the problems of pathogenesis, comorbidity, synthropy, diagnosis, and treatment of COPD.
The purpose: to investigate the relationships between the lipid and echocardiography parameters in COPD in the frame of CAD and AH comorbidity.
Materials and methods
The study was designed according to the principle of integrativity. This article presents the results of investigating lipid spectrum parameters (total cholesterol (TC), triglycerides (TG), high density lipoproteins (HDL), low density lipoproteins (LDL), very low density lipoproteins (VLDL), atherogenic index (AI)) and their correlation with echocardiography data. The diagnosis of COPD was made in accordance with the criteria represented in the report of the GOLD working group (2018). The lung function was studied using a computer spirograph Spirokom. The European Society of Cardiology guidelines (2013) were used to verify the diagnosis of AH and CAD.
The serum levels of TC, TG, HDL, LDL and VLDL were determined by a standard biochemical method, AI was calculated.
The study of the structural and functional state of the heart was carried out with the help of echocardiography using standard methods with determination of the LV end diastolic volume (LV EDV), LV end diastolic dimensions (LV EDD), posterior wall thickness in diastole (PWD), interventricular septum thickness (IVS thickness), LV mass index (LV MI), ejection fraction (EF), pulmonary artery systolic pressure, aortic root diameter (ARD), RV end diastolic dimensions (RV EDD) on Philips HD11 ultrasound machine.
Thirty-five COPD patients with CAD and AH comorbidity were examined, 57.14 % of them were men and 42.86 % — women. The average age was 57.0 [54.0–67.0] years and the mean disease duration — 10.02 [5.0–15.0] years. Twenty-three patients had CAD and AH comorbidity, 11 patients — type 2 diabetes mellitus, 8 patients — CAD (cardiosclerosis, stable angina pectoris), and 4 patients — stage 2 AH.
The statistical analysis was performed by nonparametric methods of the Statistica 10 package. Kruskal-Wallis one-way analysis of variance was used to determine the differences between independent groups. The dependence between the variables was evaluated using the Spearman correlation coefficient (R). A cluster analysis of the obtained data was also carried out; dendrograms (trees of variable) were constructed for graphical display of the results.
Results and discussion
COPD patients were divided into 3 groups: B (n = 12), C (n =14), D (n =9). In group B, 8.33 % of patients didn’t have comorbidity, 16.6 % were diagnosed with CAD, 58.33 % — AH in combination with CAD (3 persons had postinfarction cardiosclerosis). In group C, 21.43 % of patients did not have comorbidity; 28.7 % had CAD; 50 % — AH combined with CAD. In group D, all patients had comorbidity: 22.22 % — CAD and 77.78 % — AH in combination with CAD.
The signs of atherogenic dyslipidemia (an increasing level of atherogenic lipid fractions and a decreasing level of anti-atherogenic fractions) were revealed in the whole COPD group, as well as in patients with AH and CAD comorbidity (Table 1). A comparative analysis of the lipid profile (Table 1) indicates an upward trend in LDL and AI levels in case of CAD comorbidity (as compared to the group without CAD). These differences are especially significant for total cholesterol and TG (p < 0.05). AH comorbidity is also accompanied by an upward trend in total cholesterol, TG, VLDL, LDL levels and downward — in HDL.
Comparative analysis of echocardiography data (Table 2) revealed changes in parameters of both ventricles, which are more significant in AH and CAD comorbidity. Signs of the RV, RA and LV remodeling are also revealed. Changes in pulmonary blood flow lead to an increase in pulmonary artery systolic pressure, right heart strain, in structural and functional changes of the right heart, which are more pronounced in comorbidity. It should be noted that the average value of IVS thickness exceeds the normal values in all groups of patients with COPD, regardless of the presence of comorbidity that is probably a manifestation of interventricular interaction.
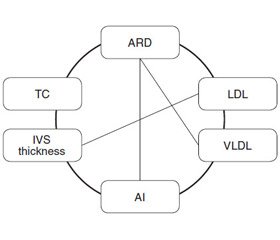
The analysis of significant correlations with the lipid profile in the group of COPD patients revealed a moderate positive correlation between TC and C-reactive protein (CRP) (R = 0.55), which indicates the lipid participation in the systemic inflammation development. Significant correlations between indices of lipid profile and echocardiogram were found, namely, TG and LV EDV (R = –0.35), LDL and IVS thickness (R = 0.41), ARD and TC (R = 0.48), LDL (R = 0.36), VLDL (R = 0.42), and AI (R = 0.36) (Fig. 1).
Significant correlations were found in group B between ARD and total cholesterol (R = 0.63), LDL (R = 0.79), VLDL (R = 0.68), between HDL and body mass index (R = 0.69), patients’ waist circumstance (R = 0.58) and between the level of bronchial obstruction reversibility with HDL (R = –0.62) and AI (R = –0.62).
In group C, there is a high positive correlation of CRP with total cholesterol (R = 0.80) and LDL (R = 0.95) that indicates the involvement of lipids in the systemic inflammation in COPD. There are negative correlations between the level of TG and ARD (R = –0.54), positive correlations between ARD and total cholesterol (R = 0.57) and VLDL (R = 0.55). Also, correlations were revealed between HDL and MPAP (R = –0.55), LDL and MPAP (R = 0.67).
Positive correlations between LDL and PWD (R = 0.71), VLDL and RV ESD (R = 0.82), as well as correlations between TG and LV EDD (R = –0.70), HDL and LV EDV (R = –0.88) were noted in group D.
In the whole COPD group, 7 positive correlations were detected between lipid profile and indicators of structural and functional state of the heart: four — between ARD and TC, LDL, VLDL, AI, one — LV EDV and TG, one — PWD with LDL and one — IVS thickness with VLDL. There are 3 positive significant correlations of ARD with TC, LDL, and VLDL in group B. There are 2 correlations with MPAP — positive with LDL and negative with HDL, as well as negative association between TG and LV EDV in group C. The correlation number increases to 6 in group D: correlations of LDL with RA, RV and LV parameters appear.
Analysis of correlation in the group without CAD comorbidity revealed 2 correlations between LDL and ARD (R = 0.85), as well as LV EDD (R = –0.83). The number of significant relationship increases with CAD comorbidity (5 correlations), namely, between total cholesterol and CRP (R = 0.50) indicating lipid participation in systemic inflammation, between ARD and total cholesterol (R = 0.43) and VLDL (R = 0.45), as well as between LDL and IVS thickness(R = 0.39), furthermore, weak positive correlation between VLDL and EF (R = 0.37), which is hardly applicable.
In COPD patients without AH, there were 3 significant correlations between structural and functional parameters of the heart and lipidogram indices: between HDL and LV EDV (R = –0.68), TG and EF (R = 0.66), as well as LV EDD (R = 0.70).
The number of correlations between lipid levels and echocardiographic data increased (5 correlations) in case of COPD with AH comorbidity, namely, between TC and ARD (R = 0.51), LDL and IVS thickness (R = 0.45), ARD (R = 0.44) and RV ESD (R = 0.54).
Therefore, lipids form a large number of correlations with various indices in COPD (anthropometry, spirometry, systemic inflammation, carbohydrate metabolism), including 9 correlations with indicators of the structural and functional state of the heart. An increase in COPD severity and AH comorbidity do not lead to an increase in the number of correlations, except for CAD when the number of correlations is two-fold increased. It is also important to note close correlation links between the lipid profile indices and the parameters of the structural and functional state of the heart. In general, the correlations were quite expected indicating lipid influence on RA, RV, LV and aortic remodeling, as well as the development of pulmonary hypertension.
Without any doubt, there is an association between RV and LV, which is due to the commonality of the anatomical structure, blood supply, interventricular septum, pericardium, the continuity of muscle syncytium, the overall effect of pressure, the chest volume and elasticity. Compensatory hyperfunction is accompanied by the fibrosis development, violation of the diastolic filling of both ventricles, and then systolic dysfunction. RV failure leads to a decrease in LV filling and in cardiac output.
The obtained data indicate a certain order of the severity of the lipid and echocardiography relationships in COPD. Apparently, these trends point out the processes of adaptation and maladaptation of the body to hypoxia that occurs and progresses in COPD.
An increase in the correlations with the so-called comorbidity may be a consequence of the synthropy between COPD, AH and atherosclerotic processes. AH in COPD is considered as pulmogenic, it develops after several years of COPD, the pathogenetic basis of AH is hypoxia [25]. Remodeling of the cardiovascular system similar to that in AH is observed before its clinical manifestation. It is emphasized that even a slight increase in blood pressure in COPD is a high additional risk of cardiovascular complications and target organ damage [25].
In order to classify the obtained data, to establish hierarchical algorithms, the cluster analysis (Fig. 2) of the structural and functional state of the myocardium and lipid spectrum was performed. Dendrograms were constructed for groups of patients with COPD as a whole, as well as for group B, C, D.
These indicators form 2 clusters, which include different echocardiography and lipid spectrum indicators. In all COPD group, the first cluster included ARD, LV EDD, RV EDD, RV ESD combined with AI and LDL. TG is relatively remote from these indicators. The second cluster includes PWD, IVS thickness and HDL, which are located close to each other and further away from the VLDL. Total cholesterol is the most distant from both clusters. It should be noted that the first cluster includes RV and LV functional parameters, and the second cluster — PWD and IVS thickness. Both clusters are combined with TG.
Cluster analysis of the indicators in groups B, C, D that differed by the severity of the patients’ condition was also carried out. In all groups, the structure of the dendrograms is preserved — there are two clusters. However, the localization of TG varies depending on the severity of COPD and comorbidity. Thus, in group B triglycerides, as well as total cholesterol act as an autonomous indicator, in group C, triglycerides become a cluster-forming index in the first cluster; in group D, triglycerides localize in the second cluster, i.e. move to the “structural” cluster. Perhaps, the reformatting of dendrograms depending on the severity of COPD reflects the processes of adaptation and maladaptation.
The similarity of dendrograms in all groups (generally COPD, COPD of groups B, C, D) reflects the unity of the echocardiography and lipid spectrum networks. Lipids, especially TG, whose role in clusters depends on the severity of the process, are the “nodes” of this network.
Already detected genes — candidates let us suggest that hypertension and atherosclerosis in patients with COPD and CAD is a manifestation of network links between lipid metabolism and myocardium and allow us to consider these phenomena within the framework of a single disease combining a phenome and a genome in diseasome.
This approach will allow us to search the drugs that affect the network metabolic “nodes” as targets at the cellular and molecular levels. This will help prevent polypragmasy, which is unavoidable in comorbidity. Statins with their pleiotropic action according to the numerous studies are discussed as such drugs [22]. The practitioner now has a difficult task: to determine empirically the phenotypic features of the disease in each patient, to evaluate the results of routine accessible research methods, anatomical, pathophysiological features, to evaluate their network relationships using the modern possibilities of the network theory, to find the signs of synthropy and to choose a pleiotropic medicinal product that will help avoid polypragmasy, by affecting many links (“nodes”, “hub”) of the network pathological process [12].
Conclusions
1. Atherogenic dyslipidemia develops in case chronic obstructive pulmonary disease with coronary artery disease and hypertension comorbidity, and is accompanied by echocardiography signs of myocardial remodeling. The revealed correlation reflects the relationship between these indicators.
2. As the severity of chronic obstructive pulmonary disease progresses, the number of correlations between lipid spectrum and structural and functional state of the myocardium increases according to the echocardiography data. In the whole group of patients and in group B, there are lipid links with only left ventricular echocardiography data; in group C, the correlation with systolic pressure in pulmonary artery appears, in group D — with right atrial, right ventricular, left ventricular parameters.
3. The accession of hypertension and coronary artery disease is accompanied by an increase in the number of correlations and their nature: in coronary artery disease, there are 5 links, without it, there are 2 links; in hypertension, there are 5 correlations, without it — 3.
4. Correlation and cluster analyzes suggest that the obtained data reflect the interactions of various nodes of the diseasome network and can be evaluated as signs of the synthropy of chronic obstructive pulmonary disease, hypertension and atherosclerosis.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
1. Mathers C.D. Projections of global mortality and burden of disease from 2002 to 2030 / C.D. Mathers, D. Loncar // PLoS Medicine. — 2006. — P. 209-224.
2. WHO Global status report on noncommunicable diseases 2010; accessed 09 April 2018. URL: http://apps.who.int/iris/bitstream/handle/10665/44579/9789240686458_eng.pdf?sequence=1
3. Nekrasov A.A. Heart remodeling in patients with COPD / A.A. Nekrasov, А.N. Kuznetsov, O.V. Melnichenko, I.S. Kruglova // Medical Almanac. — 2011. — № 3(16). — P. 112-115.
4. Huiard L. Cardiovascular morbidity and mortality in COPD / L. Huiard, P. Ernst, S. Suissa // Chest. — 2005. — 128. — P. 2640-2646.
5. Lange P. Cardiovascular morbidity in COPD: A study of the general population / P. Lange, R. Mogelvang, J.L. Marott [et al.] // COPD. — 2010. — 7777(1). — P. 5-10.
6. WHO Programmes. Chronic respiratory diseases, COPD burden; accessed 09 April 2018. URL: http://www.who.int/respiratory/copd/burden/en/
7. Chuchalin A.G. Chronic obstructive pulmonary disease and concomitant diseases / A.G. Chuchalin // Pulmonology. — 2008. — № 2. — P. 5-14.
8. Shirinsky V.S. Comorbid diseases as an important problem of clinical medicine / V.S. Shirinsky, I.V. Shirinsky // Siberian Medical Journal. — 2014. — 29(1). — P. 7-12.
9. Marx P. Comorbidities in the diseasome are more apparent than real: what Bayesian filtering reveals about the comorbidities of depression / P. Marx, P. Antal, B. Bolgar [et al.] // PLoS Comput. Biol. — 2017. — Vol. 13(6). — e1005487.
10. Putcha N. Comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans // N. Putcha, M.K. Han, C.H. Martinez [et al.] // Chronic. Obstr. Pulm. Dis. — 2014. — Vol. 1(1). — P 105-114.
11. Barnes P.G. Systemic manifestation and comorbidities of chronic obstructive pulmonary disease / P.G. Barnes, B.R. Celli // Eur. Respir. Rev. — 2013. — Vol. 22. — P. 454-475.
12. Vertkin A.L. Comorbidity / A.L. Vertkin, M.A. Rumyantsev, A.S. Skotnikov // Klinicheskaya Meditsina. — 2012. — № 10. — P. 4-11.
13. Mamaeva M.G. Features of left and right heart remodeling in patients with COPD, comorbid with ischemic heart disease / M.G. Mamaeva, I.V. Demko, А.Yu. Кraposhina, I.А. Solovieva // Modern Problems of Science and Education. — 2016. — № 6.
14. Andrea A.D. Echocardiography of the pulmonary circulation and right ventricular function / A.D. Andrea, R. Nalije, E. Greening [et al.] // Chest. — 2014. — Vol. 5(145). — P. 1071-1078.
15. Freixa X. Echocardiographic abnormalities in patients with COPD at their first hospital admission / X. Freixa, K. Portillo, C. Pare [et al.] // Eur. Resp. J. — 2013. — Vol. 41(4). — P. 784-791.
16. Macchia A. Unrecognised ventricular dysfunction in COPD / A. Macchia, I.J. Rodriguez, L. Moncalvo [et al.] // Eur. Respir. J. — 2012. — Vol. 39(1). — P. 51-58.
17. Gupta N.K. Echocardiographic evaluation of heart in chronic obstructive pulmonary disease patient and its correlation with the severity of disease / N.K. Gupta, R.K. Agraval, A.B. Srivastav, M.L. Ved // Lung India. — 2011. — 28(2). — P. 105-109.
18. Мalerba M. Subclinical left ventricular diastolic dysfunction in early stage of chronic obstructive pulmonary disease / M. Malerba, B. Ragnoli, M. Salameh [et al.] // Biol. Regul. Homeost. Agents. — 2011. — 25(3). — P. 443-451.
19. Kozlov E.V. Structural and functional changes of cardiovascular system in patients with arterial hypertension and chronic obstructive pulmonary disease / Kozlov E.V. // Siberian Medical Review. — 2016. — № 3. — P. 56-62.
20. Istomina O.V. Optimization of diagnostics of endothelial dysfunction and prognostication of course of the chronic obstructive pulmonary disease with comorbid arterial hypertension: The manuscript of dissertation on achievement of scientific degree of Candidate of Medical Science in specialty 14.01.02 “Internal diseases” / O.V. Istomina. — Kharkiv, 2018. — 17 p.
21. Feschenko Ju.I. Chronic obstructive pulmonary disease: new shades of problem: Monograph / Ju.I. Feschenko, Ju.B. Tchaikovsky, M.M. Ostrovsky, O.I. Deltsova, S.B. Gerashchenko [et al.]. — Ivano-Frankivsk: SIMIK, 2016. — 397 p.
22. Goh R.I. Exploring the human diseasome network / R.I. Goh, I.G. Choi // Briefings in Functional Genomics. — 2012. — Vol. 11(6). — P. 533-542.
23. Hobbs B.D. Integrative genomics of chronic obstructive pulmonary disease / B.D. Hobbs, C.P. Hersh // Biochem. Biophys. Res. Commun. — 2014. — Vol. 452(2). — P. 276-286.
24. Lamontagne M. Genetic regulation of gene expression in the lung identifies CST3 and CD22 as potential genes for airflow obstruction / M. Lamontagne, W. Timens, K. Hao // Thorax. — 2014. — Vol. 69(11). — P. 997-1004.
25. Akramova E.G. Complex ultrasound and functional examination of the cardiovascular system in chronic obstructive pulmonary disease: The manuscript of dissertation on achievement of scientific degree of Doctor of Medical Science in specialty 14.01.13 “Radiation diagnostics, radiation therapy” / E.G. Akramova. — Moscow, 2014. — 39 p.

/85-1.jpg)
/86-1.jpg)
/86-2.jpg)