Журнал «Актуальная инфектология» Том 8, №5, 2020
Вернуться к номеру
Клінічний випадок і літературний огляд патогенетичних аспектів комбінованої інфекції вірусу Епштейна — Барр та кандидозу порожнини рота
Авторы: S. Nykytyuk, S. Levenets, T. Kosovska, T. Nedoshytko
I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
Рубрики: Инфекционные заболевания
Разделы: Справочник специалиста
Версия для печати
Актуальність. Лікування пацієнтів iз рецидивуючою інфекцією, стійкою до традиційної терапії, є складним клінічним завданням у педіатрії. Мета роботи: проаналізувати існуючу літературу щодо клінічних особливостей кандидозу порожнини рота і інфекційного мононуклеозу. Матеріали та методи. Описаний випадок комбінованої вірусної інфекції Епштейна — Барр у формі інфекційного мононуклеозу і кандидозу ротової порожнини рота в 4-річного хлопчика. Результати. Досліджено бактеріальну флору горла і рота, в культурах iз горла були ізольовані Str.viridans 103 КУО/мл, S.aureus 105 КУО/мл, Candida albicans 106 КУО/мл. Імуноферментний аналіз показав, що індекс антитіл EBV VCA IgM становив 2,63 (більше 0,8 — позитивний результат). Висновки. При інфекційному мононуклеозі в поєднанні з кандидозом порожнини рота спостерігаються такі загальні симптоми: тривала лихоманка, лімфаденопатія і синдром екзантеми. Зазвичай вони виникають після прийому антибактеріальних засобів широкого спектра дії. У разі захворювання з такими симптомами, як тривала лихоманка, лімфаденопатія, тонзиліт і синдром екзантеми, що є спільними для кандидозу порожнини рота та інфекційного мононуклеозу, потрібна диференційна діагностика. Необхідно вивчити мікробіоту ротоглотки в пацієнтів з інфекційним мононуклеозом для виявлення флори, що може обумовлювати ускладнений перебіг захворювання і труднощі діагностики. У такому випадку цей вид дослідження є важливим діагностичним методом виявлення кандидозу.
Актуальность. Лечение пациентов с рецидивирующей инфекцией, которая устойчива к традиционной терапии, является сложной клинической задачей в педиатрии. Цель работы: проанализировать существующую литературу о клинических особенностях кандидоза полости рта и инфекционного мононуклеоза. Материалы и методы. Представлен случай комбинированной вирусной инфекции Эпштейна — Барр в форме инфекционного мононуклеоза и кандидоза ротовой полости рта у 4-летнего мальчика. Результаты. Исследована бактериальная флора горла и рта, в культурах из горла были изолированы Str.viridans 103 КОЕ/мл, S.aureus 105 КОЕ/мл, Candida albicans 106 КОЕ/мл. Иммуноферментный анализ показал, что индекс антител EBV VCA IgM составлял 2,63 (более 0,8 — положительный результат). Выводы. При инфекционном мононуклеозе в сочетании с кандидозом полости рта наблюдаются следующие общие симптомы: длительная лихорадка, лимфаденопатия и синдром экзантемы. Обычно они возникают после приема антибактериальных средств широкого спектра действия. В случае заболевания с такими симптомами, как длительная лихорадка, лимфаденопатия, тонзиллит и синдром экзантемы, которые являются общими для кандидоза полости рта и инфекционного мононуклеоза, требуется дифференциальная диагностика. Необходимо изучить микробиоту ротоглотки у пациентов с инфекционным мононуклеозом для выявления флоры, которая может обусловливать осложненное течение заболевания и трудности диагностики. В таком случае данный вид исследования является важным диагностическим методом обнаружения кандидоза.
Background. Patients with a recurrent infection that is resistant to traditional therapy are a clinical challenge in pediatrics. This recurrent pathology is often hiding another disease. In addition, timely diagnosis and suggestion of etiotropic therapy are often delayed when medical ethics have been deviated, namely, when medical history collection was dubious. The objective of this article was to present the clinical peculiarities of the Epstein-Barr virus (EBV) association with oral candidiasis. Materials and methods. The authors describe the clinical case of Epstein-Barr viral infection in a 4-year-old child. The case was detected in Ternopil region. Results. The bacterial flora of the throat and mouth was inoculated, in the throat culture, Str.viridans 103 CFU/ml, S.aureus 105 CFU/ml, Candida albicans 106 CFU/ml were isolated. Enzyme-linked immunoassay showed that EBV VCA IgM antibody index was at the level of 2.63 (more than 0.8 — a positive result). Conclusions. When infectious mononucleosis is combined with oral candidiasis, the following common symptoms are observed: prolonged fever, lymphadenopathy, and exanthema syndrome. They usually occur after administration of broad-spectrum antibacterial agents. In case of disease with symptoms such as prolonged fever, lymphadenopathy, tonsillitis, and exanthema syndrome, which are common for both oral candidiasis and infectious mononucleosis, differential diagnosis is required. There is a need to examine the microbiota of the oropharynx in patients with infectious mononucleosis to detect flora, which may lead to complicated course of the disease and diagnosis. In such case, inoculation of oropharynx microbiota is essential instrument for candidiasis diagnosis.
діти; вірусна інфекція Епштейна — Барр; кандидоз
дети; вирусная инфекция Эпштейна — Барр; кандидоз
children; Epstein-Barr viral infection; candidiasis
Introduction
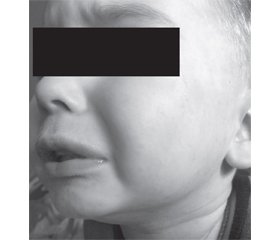
Case report
/7.jpg)
/8.jpg)
Discussion
Conclusions
- Voloha A.P. Epstein-Barr viral infection in children. Sovremennaya pediatriya. 2015. 4(68). 103-110 (in Ukrainian).
- Linke-Serinsöz E., Fend F., Quintanilla-Martinez L. Human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV) related lymphomas, pathology view point. Semin. Diagn. Pathol. 2017. 34(4). 352-363.
- Chan C.W., Chiang A.K.S., Chan K.H. Epstein-Barr virus-associated infectious mononucleosis in Chinese children. Pediatric Infectious Disease Journal. 2003. 22(11). 974-978.
- Steinbach W.J., Roilides E., Berman D. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatric Infectious Disease Journal. 2012. 31(12). 1252-1257.
- Manfredi R., Coronado O.V., Mastroianni A. Concurrent infectious mononucleosis and measles: a potentially life-threatening association sharing underlying immunodeficiency. Pediatric Infectious Disease Journal. 2003. 22(5). 470-471.
- Linde A., Falk K.I. Epstein-Barr virus. In: Manual of clinical microbiology. Ninth edition. Ed. by E.J. Barron, J.H. Jorgensen, M.L. Landry et al. ASM Press, 2007. 1564-1573.
- Setoh J.W.S., Ho C.K.M., Yung C.F. Epstein-Barr virus seroprevalence and force of infection in a multiethnic pediatric cohort, Singapore. Pediatric Infectious Disease Journal. 2019. 38(12). 1173-1176.
- Palazzi D.L., Arrieta A., Castagnola E. Candida speciation, antifungal treatment and adverse events in pediatric invasive candidiasis: results from 441 infections in a prospective, multi-national study. Pediatric Infectious Disease Journal. 2014. 33(12). 1294-1296.
- Pitetti R.D., Laus S., Wadowsky R.M. Clinical evaluation of a quantitative real time polymerase chain reaction assay for diagnosis of primary Epstein-Barr virus infection in children. Pediatric Infectious Disease Journal. 2003. 22(8). 736-739.
- Kramarev S.O., Vygovskaya O.V. Chronic forms of Epstein-Barr viral infection in children: current approaches to diagnosis and treatment. Sovremennaya pediatriya. 2008. 2(19). 103-107 (in Ukrainian).
- Martin J.M., Macias-Parra M., Mudry P. et al. Safety, efficacy, and exposure-response of voriconazole in pediatric patients with invasive aspergillosis, invasive candidiasis or esophageal candidiasis. Pediatric Infectious Disease Journal. 2017. 36(1). e1-e13.
- Lorenz M.C., Bender J.A., Fink G.R. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryotic Cell. 2004. 3(5). 1076-87.
- Autmizguine J., Tan S., Cohen-Wolkowiez M. et al. Antifungal susceptibility and clinical outcome in neonatal candidiasis. Pediatric Infectious Disease Journal. 2018. 37(9). 923-929.
- Fugl A., Andersen C.L. Epstein-Barr virus and its association with disease — a review of relevance to general practice. BMC Fam. Pract. 2019. 20. 62.
- Dunmire S.K., Hogquist K.A., Balfour H.H. Infectious mononucleosis. Curr. Top. Microbiol. Immunol. 2015. 390. 211-40.
- Sydnor E. Hospital epidemiology and infection control in acute-care settings. Clinical Microbiology Reviews. 2011. 24(1). 141-173.
- Azadmanesh J., Gowen A.M., Creger P.E., Schafer N.D., Blankenship J.R. Filamentation involves two overlapping, but distinct, programs of filamentation in the pathogenic fungus Candida albicans. G3: Genes, Genomes, Genetics. 2017. 7(11). 3797-3808.
- Grinstein S., Hube B., Mogavero S., Moran G., Westman J. Candida albicans hyphal expansion causes phagosomal membrane damage and luminal alkalinization. mBio. 2018. 9(5). e01226-18.
- Millsop J.W., Faze L.N. Oral candidiasis. Clin. Dermatol. 2016. 34. 487-494.
- Sherman R.G., Prusinski L., Joralmon R.A. Oral candidosis. Quintessence International. 2002. 33(7). 521-32.
- Vila T., Sultan A.S., Montelongo-Jauregui D., Jabra-Rizk M.A. Oral candidiasis: a disease of opportunity. Journal of Fungi. 2016. 6(1). 15.
- Kumamoto C.A. Candida biofilms. Current Opinion in Microbiology. 2002. 5(6). 608-11.
- Ortora G.J. Microbiology: an introduction. San Francisco, CA: Pearson Benjamin Cummings, 2010. 759.
- Peric M., Živkovic R., Milic Lemic A., Radunovic M., Milicic B., Arsic Arsenijevic V. The severity of denture stomatitis as related to risk factors and different Candida spp. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018. 126. 41-47.
- Singh R., Chakrabarti A. Invasive candidiasis in the Southeast-Asian region. In: Prasad R. (ed.). Candida albicans: cellular and molecular biology. 2nd ed. Switzerland: Springer International Publishing AG, 2017.
- Pfaller M.A., Diekema D.J. Epidemiology of invasive candidiasis: a persistent public health problem. Clinical Microbio-logy Reviews. 2007. 20(1). 133-63.
- Roilides E., Carlesse F. et al. Safety, efficacy and pharmacokinetics of anidulafungin in patients 1 month to < 2 years of age with invasive candidiasis, including candidemia. Pediatric Infectious Disease Journal. 2020. 39(4). 305-309.
- Tsekoura M., Ioannidou M., Pana Z.-D. Efficacy and safety of echinocandins for the treatment of invasive candidiasis in children: a meta-analysis. Pediatric Infectious Disease Journal. 2019. 38(1). 42-49.
- Benjamin D.K. Jr, Kaufman D.A., Hope W.W. A phase 3 study of micafungin versus amphotericin B deoxycholate in infants with invasive candidiasis. Pediatric Infectious Disease Journal. 2018. 37(10). 992-998.
- Kovanda L.L., Walsh T.J., Benjamin D.K. Jr. Exposure-response analysis of micafungin in neonatal candidiasis: pooled analysis of two clinical trials. Pediatric Infectious Disease Journal. 2018. 37(6). 580-585.
- Roilides E., Carlesse F., Leister-Tebbe H. A prospective, open-label study to assess the safety, tolerability and efficacy of anidulafungin in the treatment of invasive candidiasis in children 2 to < 18 years of age. Pediatric Infectious Disease Journal. 2019. 38(3). 275-279.
- Shkalim-Zemer V., Levi I., Fischer S. Response of symptomatic persistent chronic disseminated candidiasis to corticosteroid therapy in immunosuppressed pediatric patients: case study and review of the literature. Pediatric Infectious Disease Journal. 2018. 37(7). 686-690.
- Mason K.L., Erb Downward J.R., Mason K.D., Falkowski N.R., Eaton K.A., Kao J.Y., Young V.B., Hunagle G.B. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 2012. 80. 3371-3380.
- McIntyre G.T. Oral candidosis. Dent. Update. 2001. 28. 132-139.
- American Academy of Pediatrics, Committee on Infectious Diseases, Committee on Nutrition. Consumption of raw or unpasteurized milk and milk products by pregnant women and children. Pediatrics. 2014. 133(1). 175-179.