Журнал «Почки» Том 10, №4, 2021
Циклоспоринова нефропатія, її патогенез і лікування
Резюме
Циклоспорин А, отриманий із гриба під назвою Tolypocladium inflatum, почав застосовуватися в медицині в 1983 році. З початком застосування циклоспорину продемонстровано підвищення 3- та 5-річного виживання трансплантата. Однак нефротоксичність, виявлена в ранній та пізній період, ускладнює його застосування. Дуже важливо відрізняти ранню токсичність від нападів відторгнення, що пов’язано з абсолютно різними методами лікування обох процесів. Хоча вазоконстрикція в системі ниркових артерій проявляється в ранньому періоді, основним фактором пізньої токсичності є потовщення артеріолярної інтими та, як наслідок, зменшення оксигенації тканин. У статті розглянуті варіанти токсичності, зумовлені використанням циклоспорину А. На моделях щурів показані морфологічні зміни при використанні циклоспорину А. Також наведені результати власних спостережень щодо використання простагландину, який продемонстрував ефект вазодилатації, що, ймовірно, можна використовувати для подальших досліджень з метою зменшення нефротоксичності циклоспорину А. Зокрема, ми виявили, що PGE2 значно зменшує звуження судин і знижує токсичний ефект, обумовлений циклоспорином А. Обмеженням було одноразове використання цих засобів, тому ми не могли продовжувати дослідження та вводили їх тільки внутрішньовенно. Однак отримані результати виявилися обнадійливими.
Циклоспорин А, полученный из грибка под названием Tolypocladium inflatum, стал использоваться в медицине в 1983 году. С началом применения циклоспорина продемонстрировано повышение 3- и 5-летней выживаемости трансплантата. Однако нефротоксичность, наблюдаемая в раннем и позднем периодах, затрудняет его использование. Очень важно отличать раннюю токсичность от приступов отторжения, что связано с совершенно разными методами лечения обоих процессов. Хотя вазоконстрикция в системе почечных артерий проявляется в раннем периоде, основным фактором поздней токсичности является утолщение интимы артериол и, как следствие, снижение оксигенации тканей. В статье рассмотрены варианты токсичности, обусловленные использованием циклоспорина А. На моделях крыс показаны морфологические изменения при использовании циклоспорина А. Также приведены результаты собственных наблюдений по использованию простагландина, который продемонстрировал эффект вазодилатации, что, вероятно, можно применять для дальнейших исследований с целью уменьшения нефротоксичности циклоспорина А. В частности, мы обнаружили, что PGE2 значительно уменьшает сужение сосудов и снижает токсический эффект, вызванный циклоспорином А. Ограничением было однократное использование этих препаратов, поэтому мы не могли продолжать исследование и вводили их только внутривенно. Однако полученные результаты оказались обнадеживающими.
CsA, obtained from a fungus called Tolypocladium inflatum came into medical use in 1983. Organ transplants have shown great success after the use of Cyclosporine, especially in 3- and 5-year graft survival. However, nephrotoxicity seen in the early and late periods complicates its use. It is very important to distinguish especially early toxicity from rejection attacks; because the treatments of both processes are completely different. While vasocostriction in the renal artery system is prominent in the early period, the underlying factor for late toxicity is the thickening of the arteriolar intima and the consequent decrease in tissue oxygenation. The article discusses the variants of toxicity caused by the use of cyclosporin A. Morphological changes with the use of cyclosporin A are shown in rat models. The results of our own observations on the use of prostaglandin, which demonstrated the effect of vasodilation, are also presented, which can probably be used for further studies in order to reduce the nephrotoxicity of cyclosporin A. In particular, we found that PGE2 significantly reduced vasoconstriction and reduced the toxic effect due to CsA. The limitations was the usage of these agents once, so we couldn’t continue and only gave them intravenously. However, the results obtained were found to be significant.
Ключевые слова
трансплантація; токсичність циклоспорину А; вазоконстрикція артеріол нирок; нефротоксичність
трансплантация; токсичность, обусловленная циклоспорином А; вазоконстрикция почечных артериол; нефротоксичность
transplantation; cyclosporine А toxicity; renal arteriolar vasoconstriction; nephrotoxicity
CsA, is an effective immunsuppressive agent of fungal origin being widely used throughout the world since the beginning of the eighties. Prevention of the rejection attacks as well as graft versus host reactions, a low myelotoxicity and very seldom occurrence of bacterial and fungal infections during the treatment are its leading superior properties. But unfortunately, its potential nephrotoxicity during acute and chronic periods prevents its widespread and confident administration [1]. The damage formed by the use of CsA during the acute period is explained by its negative effect on renal circulation [2].
In experimental studies, it has been shown that the urinary excretion of thromboxane B2, which is a strong vasoconstrictor, increases and the production of PGE2, which is a vasodilator, decreases with the use of CsA [3–6]. In a study conducted in rats, narrowing of afferent arterioles was demonstrated by electron microscopy in kidney biopsies taken from rats given CsA [7]. Accordingly, the toxic effect of CsA is thought to arise as a result of its vasoconstriction effect [8, 9]. As a result of vasoconstriction, tissue perfusion is impaired at the microcirculation level and adversely affects kidney functions.
Since CsA is metabolized in the liver via the P450 cytochrome enzyme system, it interacts with many drugs that alter this enzyme system. Ketoconazole, cimetidine, erythromycin and corticosteroids increase blood levels of CsA by inhibiting P450 enzyme. Phenytoin, rifampicin, isoniazid, and trimethoprim decrease CsA levels by inducing P450 [10–12].
The nephrotoxic effect of CsA is enhanced when Amphotericin B is co-administered with aminoglycosides, melphalan and Co-trimoxasole [12].
Side effects related to CsA detected in clinical studies are as follows:
a) dose-dependent:
— renal dysfunction, impaired liver function, hypertension, hypertrichosis, tremor and gingival hyperplasia [12, 13];
b) dose-independent:
— paraesthesia, nausea, encephalopathy, hyperkalaemia, anaemia and susceptibility to infections [12, 13].
In addition, there is no difference between CsA and conventional immunosuppressive therapy in terms of the risk of developing lymphoma and non-lymphoma malignant diseases [13, 14].
CsA toxicity varies with dose, individual susceptibility and certain risk factors. CsA-related lesions:
1) 0–19 % acute renal failure with diffuse interstitial fibrosis;
2) 0–5 % peritubular capillary congestion;
3) 9–37 % tubular toxicity;
4) 5–30 % striped form interstitial fibrosis (interstitial areas of fibrosis in lines and atrophic tubules in these areas);
5) 5–27 % CsA-induced arteriolopathy [13].
Acute renal failure with diffuse interstitial fibrosis can be seen at any time, although peritubular congestion and tubular toxicity are more common in the first months after transplantation. CsA-induced arteriolopathy is usually seen 2 months after transplantation, and striped form interstitial fibrosis 6 months and later [13] (fig. 1).
A) Functional toxicity.
A slight decrease in renal function can be observed in almost all patients with daily administration of 10 mg/kg oral CsA after transplantation. The serum creatinine level is slightly increased. It returns to normal with decreasing the CsA dose [13]. Hypertension develops in half of the patients [15]. CsA is thought to exert a selective vasoconstriction on renal vessels, particularly on arterioles [13]. Its mechanism has been tried to be explained in four ways:
1) direct effect on renal vessels [13];
2) adrenergic system stimulation [16–18];
3) stimulation of the tubuloglomerular feedback mechanism [19, 20];
4) effects on renal prostaglandin synthesis [3, 13].
B) Tubular toxicity.
The findings are not different from those seen in functional nephrotoxicity. But the serum creatinine level is higher, it can be more than twice the normal [13, 15].
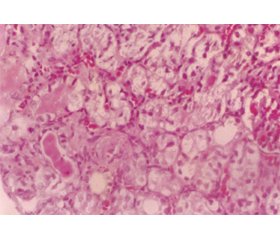
Giant mitochondria, isometric vacuolization and microcalcifications are remarkable histopathological findings. These are nonspecific, but rather characteristic lesions [13] (fig. 2).
The main etiological factor in the development of tubular toxicity is high CsA levels. This level is usually above 200 ng/ml [15].
C) Vascular-interstitial toxicity.
The patient has a slow but progressive deterioration in kidney function, resulting in hypertension. Initially, the glomerular filtration rate and renal plasma flow decrease. Then serum creatinine increases; proteinuria is mild or absent. With the discontinuation of CsA, some patients may have an improvement in renal function, but most do not. As a sign of vascular damage, some patients have increased plasma levels of endothelial-derived factor VIII and antithrombin [13, 15].
Renal vasoconstriction is shown as the main cause of CsA-induced nephrotoxicity (fig. 3). For this reason, the first thing that comes to mind for the prevention or treatment of renal toxicity is to reduce the dose of CsA or to replace it with another immunosuppressive. Although FK506-induced nephrotoxicity is observed, it is not as severe as CsA as is known. That’s why Tacrolimus seems like an option. The newly introduced co-stimulator blockers are another option. However, the high risk of infection in these should not be ignored.
In a study we conducted on rats, we tried PGI2 (prostacyclin) and PGE2, which have vasodilator effects, in this context. In particular, we found that PGE2 significantly reduced vasoconstriction and reduced the toxic effect due to CsA. Our problem here was that we only used these agents once, so we couldn’t continue and only gave them intravenously. However, the results obtained were found to be significant.
Received 25.09.2021
Revised 02.10.2021
Accepted 10.10.2021
Список литературы
1. Cohen D.J., Loertscher R., Rubin M.F., Tilney N.L., Carpenter C.B., Strom T.B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann. Int. Med. 1984. 101. 667-682.
2. Kahan B.D. Cyclosporine. In: Massry S.G., Glassock R.S., eds. Textbook of Nephrology. 2nd ed. Baltimore: Williams&Wilkins, 1989. 1487-1499.
3. Faustman D.L., Hauptfeld V., Davie J.M., Lacy P.E. Increase in urinary thromboxane B2 in rats caused by Cyclosporine. Transplant. 1985. 40. 2. 214-217.
4. Petric R., Freeman D., Wallace C., McDonald J., Stiller C., Keown P. Effect of cyclosporine on urinary prostanoid excretion, renal blood flow, and glomerulotubular function. Transplant. 1988. 45. 5. 883-889.
5. Petric R., Freeman D., Wallace C., Stiller C., Keown P. Amelioration of experimental cyclosporine nephrotoxicity by calcium channel inhibition. Brief. Communications. 1992. 1103-1105.
6. Stahl R.A.K., Kudelka S. Chronic cyclosporine A treatment reduces prostaglandin E2 formation in isolated glomeruli and papilla of rat’s kidneys. Clin. Nephrol. 1986. 25. 1. 78-82.
7. English J., Evan A., Houghton D.C., Bennett W.M. Cyclosporine-induced acute dysfunction in the rat. Transplant. 1987. 44. 1. 135-141.
8. Moss N.G., Powell S.L., Falk R.J. Intravenous cyclosporine activates afferent and efferent renal nerves and causes sodium retention in innervated kidneys in rats. Proc. Natl. Acad. Sci. 1985. 82. 8222-8226.
9. Murray B.M., Paller M.S. Beneficial effects of renal denervation and prazosin on GFR and renal blood flow after cyclosporine in rats. Clin. Nephrol. 1986. 25. 1. 37-39.
10. Dieperink H., Kemp E., Leyssac P.P. et al. Ketoconazole and cyclosporine A: combined effects on rat renal function and on serum and tissue cyclosporine A concentration. Clin. Nephrol. 1986. 25. 1. 137-143.
11. Henricsson S., Lindholm A., Aravoglou M. Cyclosporin metabolism in human liver microsomes and its inhibition by other drugs. Pharmacol. Toxicol. 1990. 66. 1. 49-52.
12. Mihatsch M.J., Ryffel B., Gudat F., Thiel G. Cyclosporine nephropathy. In: Tisher C.C., Brenner B.M., eds. Renal pathology with clinical and functional correlations. Vol. II. Philadelphia: J.B. Lippincott, 1989. 1555-1586.
13. Hamilton D.V., Evans D.B., Thiru S. Toxicity of cyclosporine A in organ grafting. In: Cyclosporin A. Oxford, 1982. 393-411.
14. Winkelstein A. Immunosuppressive therapy. In: Stites D.P., Terr A.I., eds. Basic and Clinical Immunulogy. 7th ed. Connecticut: Appleton and Lange, 1991. 775.
15. Mihatsch M.J., Thiel G., Ryffel B. Morphologic diagnosis of cyclosporine nephrotoxixicy. Seminars in Diagnostic Pathology. 1988. 5. 1. 104-121.
16. Cotran R.S., Kumar V., Robbins S.L. Diseases of immunity. In: W.B. Saunders Staff ed. Robbins pathologic basis of disease. 4th ed. Philadelphia: W.B. Saunders, 1989. 163-237.
17. Garr M.D., Paller M.S. Cyclosporine augments the renal vasoconstrictor response to norepinephrine. Am. J. Phisiol. 1990. 1. 211-7.
18. Owen M. Major histocompatibility complex. In: Roitt I.V., Brostoff J., Male D.K. Immunology. 2nd ed. New York: Gower Medical Publishing, 1989. 4. 1-4.11.
19. Mihatsch M.J., Morozumi K., Ryffel B., Thiel G. Old and new aspects of cyclosporine nephropathy. XIX International Congress of the International Academy of Pathology. Madrid, 1992.
20. Schachter M. Cyclosporine A and hypertension. Journal of Hypertension. 1988. 6. 511-516.
/12.jpg)

/11.jpg)