Журнал "Гастроэнтерология" Том 59, №3, 2025
Вернуться к номеру
Внутрішньоклітинний кальцій у підшлунковій залозі. Частина 2. Роль при гострому панкреатиті
Авторы: Чуклін С.М. (1), Чуклін С.С. (1), Бариляк Р.В. (2)
(1) - Медичний центр Святої Параскеви, м. Львів, Україна
(2) - Львівська обласна клінічна лікарня, м. Львів, Україна
Рубрики: Гастроэнтерология
Разделы: Справочник специалиста
Версия для печати
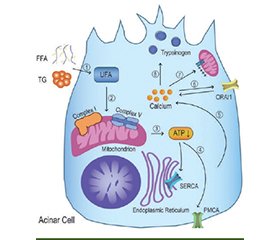
Актуальність. Порушення кальцієвого гомеостазу є центральною ланкою патогенезу гострого панкреатиту (ГП). Надлишкове накопичення Ca2+ у клітинах підшлункової залози ініціює каскад ушкоджень, що охоплює активацію зимогенів, мітохондріальну дисфункцію, енергетичне виснаження, стрес ендоплазматичного ретикулуму, некроз, апоптоз і розвиток запальної відповіді. Мета: проаналізувати сучасні дані щодо внутрішньоклітинної кальцієвої дисрегуляції при ГП з урахуванням ролі кальцієвої сигналізації в ушкодженні ацинарних, протокових, зірчастих і імунних клітин. Матеріали та методи. Проведено аналіз публікацій, відібраних з баз даних PubMed, Scopus і Google Scholar. Основну увагу приділено дослідженням кальцієвого обміну в ацинарних і протокових клітинах, кальцієвим каналам (Orai1, Piezo1, TRPV4), механізмам взаємодії з імунною системою. Результати. Встановлено, що внутрішньоклітинна кальцієва дисрегуляція є ключовою патофізіологічною подією при ГП. В ацинарних клітинах надмірне надходження Ca2+ активує іонні канали Orai1 та TRPV4, що сприяє хронічному підвищенню цитозольної концентрації кальцію. Це порушує функцію Ca2+-АТФаз, унеможливлює кліренс кальцію з цитозолю та створює умови для активації внутрішньоклітинних ферментів — насамперед трипсиногену. Кальцієве перевантаження мітохондрій призводить до відкриття перехідної пори мітохондрій, зниження мембранного потенціалу, зменшення продукції АТФ та запуску апоптозу або некрозу. У клітинах проток підшлункової залози патологічні коливання Ca2+ знижують експресію щільних контактів (ZO-1, E-кадгерин), підвищують проникність епітеліального бар’єра та створюють умови для трансдукції запального сигналу. У протокових клітинах перевантаження Ca2+ спричиняє пригнічення секреції рідини та HCO3–, порушення мітохондріальної функції та некроз. У зірчастих клітинах підшлункової залози кальцієві сигнали сприяють активації фібробластичного фенотипу з посиленою експресією TGF-β1, фібронектину та колагену І типу. Не менш важливою є кальцієво-опосередкована активація імунних клітин, зокрема макрофагів, що сприяє секреції прозапальних цитокінів і підтриманню системного запального каскаду. Висновки. Дисбаланс кальцію є критичним фактором ушкодження тканини підшлункової залози при ГП. Удосконалення розуміння кальцієвих сигналів відкриває перспективи для розробки нових методів лікування, спрямованих на запобігання прогресуванню та ускладненням захворювання.
Background. Disruption of calcium homeostasis is a central mechanism in the pathogenesis of acute pancreatitis (AP). Excessive accumulation of Ca2+ in pancreatic cells initiates a cascade of injuries, including zymogen activation, mitochondrial dysfunction, energy depletion, endoplasmic reticulum stress, necrosis, apoptosis, and the development of an inflammatory response. Objective: to analyze current data on intracellular calcium dysregulation in AP, with a focus on the role of calcium signaling in injury to acinar, ductal, stellate, and immune cells. Materials and methods. A literature review was conducted using PubMed, Scopus, and Google Scholar databases. The analysis focused on calcium metabolism in acinar and ductal cells, calcium channels (Orai1, Piezo1, TRPV4), and mechanisms of interaction with the immune system. Results. Intracellular calcium dysregulation is a key pathophysiological event in AP. In acinar cells, excessive Ca2+ influx via Orai1 and TRPV4 ion channels leads to sustained elevation of cytosolic calcium. This impairs Ca2+-ATPase activity, hampers effective calcium clearance from the cytosol, and promotes premature activation of intracellular enzymes, primarily trypsinogen. Mitochondrial calcium overload triggers the opening of the mitochondrial permeability transition pore, reduces membrane potential, decreases ATP production, and initiates apoptosis or necrosis. In pancreatic duct cells, pathological Ca2+ oscillations reduce the expression of tight junctions (ZO-1, E-cadherin), increase the permeability of the epithelial barrier, and create conditions for inflammatory signal transduction. In ductal cells, Ca2+ overload suppresses fluid and HCO3– secretion, impairs mitochondrial function, and induces necrosis. In pancreatic stellate cells, calcium signaling promotes activation of fibroblast phenotype characterized by increased expression of transforming growth factor β1, fibronectin, and type I collagen. Calcium-mediated activation of immune cells is equally important, particularly macrophages, which contributes to the release of proinflammatory cytokines and perpetuates the systemic inflammatory cascade. Conclusions. Calcium imbalance is a critical factor in pancreatic tissue damage in AP. Advancing the understanding of calcium signaling offers new perspectives for the development of new treatment strategies aimed at preventing disease progression and complications.
внутрішньоклітинний кальцій; кальцієва сигналізація; підшлункова залоза; гострий панкреатит
intracellular calcium; calcium signaling; pancreas; acute pancreatitis
Для ознакомления с полным содержанием статьи необходимо оформить подписку на журнал.
- Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyin–goh D, et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology. 2022 Jan;162(1):122-134. doi: 10.1053/j.gastro.2021.09.043. Epub 2021 Sep 25. PMID: 34571026.
- Li CL, Jiang M, Pan CQ, Li J, Xu LG. The global, regional, and national burden of acute pancreatitis in 204 countries and territories, 1990-2019. BMC Gastroenterol. 2021 Aug 25;21(1):332. doi: 10.1186/s12876-021-01906-2. PMID: 34433418.
- Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019 Aug;16(8):479-496. doi: 10.1038/s41575-019-0158-2. PMID: 31138897.
- Qiu M, Zhou X, Zippi M, Goyal H, Basharat Z, et al. Comprehensive review on the pathogenesis of hypertriglyceridaemia-associated acute pancreatitis. Ann Med. 2023;55(2):2265939. doi: 10.1080/07853890.2023.2265939. Epub 2023 Oct 9. PMID: 37813108.
- Boškoski I, Costamagna G. How to Prevent Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Gastroenterology. 2020 Jun;158(8):2037-2040. doi: 10.1053/j.gastro.2020.03.019. Epub 2020 Mar 18. PMID: 32197979.
- Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. 2020 Sep 5;396(10252):726-734. doi: 10.1016/S0140-6736(20)31310-6. PMID: 32891214.
- Luo T, Tang Y, Xie W, Ma Z, Gong J, Zhang Y, et al. Cerium-based nanoplatform for severe acute pancreatitis: Achieving enhanced anti-inflammatory effects through calcium homeostasis restoration and oxidative stress mitigation. Mater Today Bio. 2025 Jan 13;31:101489. doi: 10.1016/j.mtbio.2025.101489. eCollection 2025 Apr. PMID: 39906206.
- Kang H, Yang Y, Zhu L, Zhao X, Li J, et al. Role of neutrophil extracellular traps in inflammatory evolution in severe acute pancreatitis. Chin Med J (Engl). 2022 Dec 5;135(23):2773-2784. doi: 10.1097/CM9.0000000000002359. PMID: 36729096.
- Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, et al. NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis. Gastroenterology. 2020 Jan;158(1):253-269.e14. doi: 10.1053/j.gastro.2019.09.040. Epub 2019 Oct 5. PMID: 31593700.
- Habtezion A, Gukovskaya AS, Pandol SJ. Acute Pancrea–titis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology. 2019 May;156(7):1941-1950. doi: 10.1053/j.gastro.2018.11.082. Epub 2019 Jan 18. PMID: 30660726.
- Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, et al. Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models. Gastroenterology. 2018 Feb;154(3):689-703. doi: 10.1053/j.gastro.2017.10.012. Epub 2017 Oct 23. PMID: 29074451.
- Huang Y, Badurdeen DS. Acute Pancreatitis Review. Turk J Gastroenterol. 2023 Aug;34(8):795-801. doi: 10.5152/tjg.2023.23175. PMID: 37404118.
- Petersen OH, Gerasimenko JV, Gerasimenko OV, Gryshchenko O, Peng S. The roles of calcium and ATP in the physiology and pathology of the exocrine pancreas. Physiol Rev. 2021 Oct 1;101(4):1691-1744. doi: 10.1152/physrev.00003.2021. Epub 2021 May 5. PMID: 33949875.
- Takano T, Yule DI. Neuronal and hormonal control of Ca2+ signalling in exocrine glands: insight from in vivo studies. J Physiol. 2024 Jul;602(14):3341-3350. doi: 10.1113/JP285461. Epub 2024 Jun 7. PMID: 38847391.
- Hegyi P, Rakonczay Z Jr. The role of pancreatic ducts in the pathogenesis of acute pancreatitis. Pancreatology. 2015 Jul;15(4 Suppl):S13-7. doi: 10.1016/j.pan.2015.03.010. Epub 2015 Apr 7. PMID: 25921231.
- Gryshchenko O, Gerasimenko JV, Peng S, Gerasimenko OV, Petersen OH. Calcium signalling in the acinar environment of the exocrine pancreas: physiology and pathophysiology. J Physiol. 2018 Jul;596(14):2663-2678. doi: 10.1113/JP275395. Epub 2018 Mar 26. PMID: 29424931.
- Pandol SJ, Gottlieb RA. Calcium, mitochondria and the initiation of acute pancreatitis. Pancreatology. 2022 Nov;22(7):838-845. doi: 10.1016/j.pan.2022.07.011. Epub 2022 Aug 3. PMID: 35941013.
- Hong WL, Huang H, Zeng X, Duan CY. Targeting mitochondrial quality control: new therapeutic strategies for major diseases. Mil Med Res. 2024 Aug 21;11(1):59. doi: 10.1186/s40779-024-00556-1. PMID: 39164792.
- Zaman S, Gorelick F. Acute pancreatitis: pathogenesis and emerging therapies. J Pancreatol. 2024 Mar;7(1):10-20. doi: 10.1097/JP9.0000000000000168. Epub 2024 Jan 2. PMID: 38524855.
- Li H, Wu D, Zhang H, Li P. New insights into regulatory cell death and acute pancreatitis. Heliyon. 2023 Jul 7;9(7):e18036. doi: 10.1016/j.heliyon.2023.e18036. eCollection 2023 Jul. PMID: 37519748.
- Gukovskaya AS, Gorelick FS, Groblewski GE, Mareninova OA, Lugea A, Antonucci L, et al. Recent Insights Into the Pathogenic Mechanism of Pancreatitis: Role of Acinar Cell Orga–nelle Disorders. Pancreas. 2019 Apr;48(4):459-470. doi: 10.1097/MPA.0000000000001298. PMID: 30973461.
- Feng S, Wei Q, Hu Q, Huang X, Zhou X, Luo G, et al. Research Progress on the Relationship Between Acute Pancreatitis and Calcium Overload in Acinar Cells. Dig Dis Sci. 2019 Jan;64(1):25-38. doi: 10.1007/s10620-018-5297-8. Epub 2018 Oct 3. PMID: 30284136.
- Garami A, Hegyi P. Precision Medicine in Pancreatitis: The Future of Acute Pancreatitis Care. Function (Oxf). 2023 Apr 5;4(3):zqad015. doi: 10.1093/function/zqad015. eCollection 2023. PMID: 37168493.
- Pallagi P, Madácsy T, Varga Á, Maléth J. Intracellular Ca2+ Signalling in the Pathogenesis of Acute Pancreatitis: Recent Advances and Translational Perspectives. Int J Mol Sci. 2020 Jun 3;21(11):4005. doi: 10.3390/ijms21114005. PMID: 32503336.
- Pallagi P, Görög M, Papp N, Madácsy T, Varga Á, Crul T, et al. Bile acid- and ethanol-mediated activation of Orai1 dama–ges pancreatic ductal secretion in acute pancreatitis. J Physiol. 2022 Apr;600(7):1631-1650. doi: 10.1113/JP282203. Epub 2022 Feb 17. PMID: 35081662.
- Waldron RT, Chen Y, Pham H, Go A, Su HY, Hu C, et al. The Orai Ca2+ channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J Physiol. 2019 Jun;597(12):3085-3105. doi: 10.1113/JP277856. Epub 2019 May 22. PMID: 31050811.
- Swain SM, Romac JM, Shahid RA, Pandol SJ, Liedtke W, et al. TRPV4 channel opening mediates pressure-induced pancreatitis initiated by Piezo1 activation. J Clin Invest. 2020 May 1;130(5):2527-2541. doi: 10.1172/JCI134111. PMID: 31999644.
- Habtezion A, Gukovskaya AS, Pandol SJ. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology. 2019 May;156(7):1941-1950. doi: 10.1053/j.gastro.2018.11.082. Epub 2019 Jan 18. PMID: 30660726.
- Sendler M, Algül H. [Pathogenesis of acute pancreatitis]. Internist (Berl). 2021 Oct;62(10):1034-1043. doi: 10.1007/s00108-021-01158-y. Epub 2021 Sep 16. PMID: 34529120. German.
- Boyman L, Karbowski M, Lederer WJ. Regulation of Mitochondrial ATP Production: Ca2+ Signaling and Quality Control. Trends Mol Med. 2020 Jan;26(1):21-39. doi: 10.1016/j.molmed.2019.10.007. Epub 2019 Nov 22. PMID: 31767352.
- Gerasimenko JV, Peng S, Tsugorka T, Gerasimenko OV. Ca2+ signalling underlying pancreatitis. Cell Calcium. 2018 Mar;70:95-101. doi: 10.1016/j.ceca.2017.05.010. Epub 2017 May 18. PMID: 28552244.
- Mititelu A, Grama A, Colceriu MC, Pop TL. Overview of the cellular and immune mechanisms involved in acute pancreatitis: In search of new prognosis biomarkers. Expert Rev Mol Med. 2025 Jan 6;27:e9. doi: 10.1017/erm.2024.40. PMID: 39757373.
- Wang Q, Bai L, Luo S, Wang T, Yang F, Xia J, et al. TMEM16A Ca2+-activated Cl- channel inhibition ameliorates acute pancreatitis via the IP3R/Ca2+/NFκB/IL-6 signaling pathway. J Adv Res. 2020 Jan 21;23:25-35. doi: 10.1016/j.jare.2020.01.006. eCollection 2020 May. PMID: 32071789.
- Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, et al. Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models. Gastroenterology. 2018 Feb;154(3):689-703. doi: 10.1053/j.gastro.2017.10.012. Epub 2017 Oct 23. PMID: 29074451.
- Yang HY, Liang ZH, Xie JL, Wu Q, Qin YY, et al. Gelsolin impairs barrier function in pancreatic ductal epithelial cells by actin filament depolymerization in hypertriglyceridemia-induced pancreatitis in vitro. Exp Ther Med. 2022 Apr;23(4):290. doi: 10.3892/etm.2022.11219. Epub 2022 Feb 16. PMID: 35317441.
- Wen L, Javed TA, Yimlamai D, Mukherjee A, Xiao X, Husain SZ. Transient High Pressure in Pancreatic Ducts Promotes Inflammation and Alters Tight Junctions via Calcineurin Signaling in Mice. Gastroenterology. 2018 Oct;155(4):1250-1263.e5. doi: 10.1053/j.gastro.2018.06.036. Epub 2018 Jun 19. PMID: 29928898.
- Yang H, Liang Z, Xie J, Wu Q, Qin Y, et al. Gelsolin inhibits autophagy by regulating actin depolymerization in pancreatic ductal epithelial cells in acute pancreatitis. Braz J Med Biol Res. 2023 Jan 27;56:e12279. doi: 10.1590/1414-431X2023e12279. eCollection 2023. PMID: 36722658.
- Maléth J, Hegyi P. Calcium signaling in pancreatic ductal epithelial cells: an old friend and a nasty enemy. Cell Calcium. 2014 Jun;55(6):337-45. doi: 10.1016/j.ceca.2014.02.004. Epub 2014 Feb 15. PMID: 24602604.
- Ferdek PE, Jakubowska MA, Gerasimenko JV, Gerasimenko OV, Petersen OH. Bile acids induce necrosis in pancreatic stellate cells dependent on calcium entry and sodium-driven bile uptake. J Physiol. 2016 Nov 1;594(21):6147-6164. doi: 10.1113/JP272774. Epub 2016 Aug 8. PMID: 27406326.
- Gryshchenko O, Gerasimenko JV, Gerasimenko OV, Peter–sen OH. Ca(2+) signals mediated by bradykinin type 2 receptors in normal pancreatic stellate cells can be inhibited by specific Ca(2+) channel blockade. J Physiol. 2016 Jan 15;594(2):281-93. doi: 10.1113/JP271468. Epub 2015 Nov 8. PMID: 26442817.
- Gryshchenko O, Gerasimenko JV, Petersen OH, Gerasimenko OV. Calcium Signaling in Pancreatic Immune Cells In situ. Function (Oxf). 2020 Oct 13;2(1):zqaa026. doi: 10.1093/function/zqaa026. eCollection 2021. PMID: 35330972.
- Solis AG, Bielecki P, Steach HR, Sharma L, Harman CCD, Yun S, et al. Mechanosensation of cyclical force by Piezo1 is essential for innate immunity. Nature. 2019 Sep;573(7772):69-74. doi: 10.1038/s41586-019-1485-8. Epub 2019 Aug 21. PMID: 31435009.
- Barreto SG, Habtezion A, Gukovskaya A, Lugea A, Jeon C, Yadav D, et al. Critical thresholds: key to unlocking the door to the prevention and specific treatments for acute pancreatitis. Gut. 2021 Jan;70(1):194-203. doi: 10.1136/gutjnl-2020-322163. Epub 2020 Sep 24. PMID: 32973069.
- Takahashi T, Miao Y, Kang F, Dolai S, Gaisano HY. Susceptibility Factors and Cellular Mechanisms Underlying Alcoholic Pancreatitis. Alcohol Clin Exp Res. 2020 Apr;44(4):777-789. doi: 10.1111/acer.14304. Epub 2020 Mar 1. PMID: 32056245.
- Petersen OH. Different Effects of Alcohol on the Liver and the Pancreas. Function (Oxf). 2021 Feb 19;2(2):zqab008. doi: 10.1093/function/zqab008. eCollection 2021. PMID: 35330811.
- Huang W, Booth DM, Cane MC, Chvanov M, Javed MA, Elliott VL, et al. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut. 2014 Aug;63(8):1313-24. doi: 10.1136/gutjnl-2012-304058. Epub 2013 Oct 25. PMID: 24162590.
- Kusiak AA, Jakubowska MA, Stopa KB, Zhang X, Huang W, Gerasimenko JV, et al. Activation of pancreatic stellate cells attenuates intracellular Ca2+ signals due to downregulation of TRPA1 and protects against cell death induced by alcohol metabolites. Cell Death Dis. 2022 Aug 29;13(8):744. doi: 10.1038/s41419-022-05186-w. PMID: 36038551.
- Żorniak M, Sirtl S, Mayerle J, Beyer G. What Do We Currently Know about the Pathophysiology of Alcoholic Pancreatitis: A Brief Review. Visc Med. 2020 Jun;36(3):182-190. doi: 10.1159/000508173. Epub 2020 Jun 10. PMID: 32775348.
- Rasineni K, Srinivasan MP, Balamurugan AN, Kaphalia BS, Wang S, Ding WX, et al. Recent Advances in Understanding the Complexity of Alcohol-Induced Pancreatic Dysfunction and Pancreatitis Development. Biomolecules. 2020 Apr 27;10(5):669. doi: 10.3390/biom10050669. PMID: 32349207.
- Parys JB, Vervliet T. New Insights in the IP3 Receptor and Its Regulation. Adv Exp Med Biol. 2020;1131:243-270. doi: 10.1007/978-3-030-12457-1_10. PMID: 31646513.
- Patel K, Durgampudi C, Noel P, Trivedi RN, de Oliveira C, Singh VP. Fatty Acid Ethyl Esters Are Less Toxic Than Their Parent Fatty Acids Generated during Acute Pancreatitis. Am J Pathol. 2016 Apr;186(4):874-84. doi: 10.1016/j.ajpath.2015.11.022. Epub 2016 Feb 12. PMID: 26878214.
- Gerasimenko J, Peng S, Gerasimenko O. Role of acidic stores in secretory epithelia. Cell Calcium. 2014 Jun;55(6):346-54. doi: 10.1016/j.ceca.2014.04.002. Epub 2014 Apr 18. PMID: 2483210.
- Dolai S, Liang T, Lam PPL, Fernandez NA, Chidambaram S, Gaisano HY. Effects of ethanol metabolites on exocytosis of pancreatic acinar cells in rats. Gastroenterology. 2012 Sep;143(3):832-843.e7. doi: 10.1053/j.gastro.2012.06.011. Epub 2012 Jun 15. PMID: 22710192.
- Gerasimenko JV, Petersen OH, Gerasimenko OV. SARS-CoV-2 S Protein Subunit 1 Elicits Ca2+ Influx-Dependent Ca2+ Signals in Pancreatic Stellate Cells and Macrophages In Situ. Function (Oxf). 2022 Jan 31;3(2):zqac002. doi: 10.1093/function/zqac002. eCollection 2022. PMID: 35284826.
- Zhang T, Chen S, Li L, Jin Y, Liu S, Liu Z, et al. PFKFB3 controls acinar IP3R-mediated Ca2+ overload to regulate acute pancreatitis severity. JCI Insight. 2024 May 23;9(13):e169481. doi: 10.1172/jci.insight.169481. PMID: 38781030.
- Madácsy T, Varga Á, Papp N, Tél B, Pallagi P, Szabó V, et al. Impaired regulation of PMCA activity by defective CFTR expression promotes epithelial cell damage in alcoholic pancreatitis and hepatitis. Cell Mol Life Sci. 2022 Apr 28;79(5):265. doi: 10.1007/s00018-022-04287-1. PMID: 35484438.
- Lerch MM, Saluja AK, Rünzi M, Dawra R, Saluja M, Steer ML. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology. 1993 Mar;104(3):853-61. doi: 10.1016/0016-5085(93)91022-a. PMID: 7680018.
- Mooren FCh, Hlouschek V, Finkes T, Turi S, Weber IA, Singh J, et al. Early changes in pancreatic acinar cell calcium signaling after pancreatic duct obstruction. J Biol Chem. 2003 Mar 14;278(11):9361-9. doi: 10.1074/jbc.M207454200. Epub 2003 Jan 8. PMID: 12522141.
- Senninger N, Moody FG, Coelho JC, Van Buren DH. The role of biliary obstruction in the pathogenesis of acute pancreatitis in the opossum. Surgery. 1986 Jun;99(6):688-93. PMID: 2424109.
- Voronina S, Longbottom R, Sutton R, Petersen OH, Tepikin A. Bile acids induce calcium signals in mouse pancreatic acinar cells: implications for bile-induced pancreatic pathology. J Physiol. 2002 Apr 1;540(Pt 1):49-55. doi: 10.1113/jphysiol.2002.017525. PMID: 11927.
- Fischer L, Gukovskaya AS, Penninger JM, Mareninova OA, Friess H, et al. Phosphatidylinositol 3-kinase facilitates bile acid-induced Ca(2+) responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007 Mar;292(3):G875-86. doi: 10.1152/ajpgi.00558.2005. Epub 2006 Dec 7. PMID: 17158252.
- Geyer N, Diszházi G, Csernoch L, Jóna I, Almássy J. Bile acids activate ryanodine receptors in pancreatic acinar cells via a direct allosteric mechanism. Cell Calcium. 2015 Aug;58(2):160-70. doi: 10.1016/j.ceca.2015.03.009. Epub 2015 Apr 17. PMID: 25931303.
- Katona M, Hegyi P, Kui B, Balla Z, Rakonczay Z Jr, Rázga Z, et al. A novel, protective role of ursodeoxycholate in bile-induced pancreatic ductal injury. Am J Physiol Gastrointest Liver Physiol. 2016 Feb 1;310(3):G193-204. doi: 10.1152/ajpgi.00317.2015. Epub 2015 Nov 25. PMID: 26608189.
- Tran QT, Tran VH, Sendler M, Doller J, Wiese M, Bolsmann R, et al. Role of Bile Acids and Bile Salts in Acute Pancreatitis: From the Experimental to Clinical Studies. Pancreas. 2021 Jan 1;50(1):3-11. doi: 10.1097/MPA.0000000000001706. PMID: 33370017.
- Romac JM, Shahid RA, Swain SM, Vigna SR, Liddle RA. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat Commun. 2018 Apr 30;9(1):1715. doi: 10.1038/s41467-018-04194-9. PMID: 29712913.
- Lai A, Cox CD, Chandra Sekar N, Thurgood P, Jaworow–ski A, et al. Mechanosensing by Piezo1 and its implications for physiology and various pathologies. Biol Rev Camb Philos Soc. 2022 Apr;97(2):604-614. doi: 10.1111/brv.12814. Epub 2021 Nov 15. PMID: 34781417.
- Auwercx J, Fourgeaud M, Lalot A, Gautier M. Unraveling the Connection Between Ion Channels and Pancreatic Stellate Cell Activation. Cell Physiol Biochem. 2025 Jan 18;59(S1):25-40. doi: 10.33594/000000754. PMID: 39825736.
- Akshintala VS, Kanthasamy K, Bhullar FA, Sperna Weiland CJ, Kamal A, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: an updated systematic review and meta-analysis of 145 randomized controlled trials. Gastrointest Endosc. 2023 Jul;98(1):1-6.e12. doi: 10.1016/j.gie.2023.03.023. Epub 2023 Mar 31. PMID: 37004815.
- Mutneja HR, Vohra I, Go A, Bhurwal A, Katiyar V, Palomera Tejeda E, et al. Temporal trends and mortality of post-ERCP pancreatitis in the United States: a nationwide analysis. Endoscopy. 2021 Apr;53(4):357-366. doi: 10.1055/a-1220-2242. Epub 2020 Sep 9. PMID: 32668463.
- Akshintala VS, Boparai IS, Barakat MT, Husain SZ. Post Endoscopic Retrograde Cholangiopancreatography Pancreatitis: Novel Mechanisms and Prevention by Drugs. United European Gastroenterol J. 2024 Dec 23. doi: 10.1002/ueg2.12732. Online ahead of print. PMID: 39711464.
- Ogura T, Imoto A, Okuda A, Fukunishi S, Higuchi K. Can Iodixanol Prevent Post-Endoscopic Retrograde Cholangiopancreato–graphy Pancreatitis? A Prospective, Randomized, Controlled Trial. Dig Dis. 2019;37(3):255-261. doi: 10.1159/000496349. Epub 2019 Jan 17. PMID: 30654370.
- Jin S, Orabi AI, Le T, Javed TA, Sah S, Eisses JF, Bottino R, Molkentin JD, Husain SZ. Exposure to Radiocontrast Agents Induces Pancreatic Inflammation by Activation of Nuclear Factor-κB, Calcium Signaling, and Calcineurin. Gastroenterology. 2015 Sep;149(3):753-64.e11. doi: 10.1053/j.gastro.2015.05.004. Epub 2015 May 14. PMID: 25980752.
- Furukawa R, Kuwatani M, Jiang JJ, Tanaka Y, Hasebe R, Murakami K, et al. GGT1 is a SNP eQTL gene involved in STAT3 activation and associated with the development of Post-ERCP pancreatitis. Sci Rep. 2024 May 28;14(1):12224. doi: 10.1038/s41598-024-60312-2. PMID: 38806529.
- Pan X, Ye L, Ren Z, Li J, Li B, et al. Biochanin A ameliorates caerulein-induced acute pancreatitis and associated intestinal injury in mice by inhibiting TLR4 signaling. J Nutr Biochem. 2023 Mar;113:109229. doi: 10.1016/j.jnutbio.2022.109229. Epub 2022 Nov 23. PMID: 36435290.
- Swain SM, Liddle RA. Mechanosensing Piezo channels in gastrointestinal disorders. J Clin Invest. 2023 Oct 2;133(19):e171955. doi: 10.1172/JCI171955. PMID: 37781915.
- Zhang D, Man X, Li L, Tang J, Liu F. Radiocontrast agent and intraductal pressure promote the progression of post-ERCP pancreatitis by regulating inflammatory response, cellular apoptosis, and tight junction integrity. Pancreatology. 2022 Jan;22(1):74-82. doi: 10.1016/j.pan.2021.11.004. Epub 2021 Nov 9. PMID: 34810073.
- Wen L, Javed TA, Dobbs AK, Brown R, Niu M, Li L, et al. The Protective Effects of Calcineurin on Pancreatitis in Mice Depend on the Cellular Source. Gastroenterology. 2020 Sep;159(3):1036-1050.e8. doi: 10.1053/j.gastro.2020.05.051. Epub 2020 May 20. PMID: 32445858.
- Akshintala VS, Husain SZ, Brenner TA, Singh A, Singh VK, Khashab MA, et al. Rectal INdomethacin, oral TacROlimus, or their combination for the prevention of post-ERCP pancreatitis (INTRO Trial): Protocol for a randomized, controlled, double-blin–ded trial. Pancreatology. 2022 Nov;22(7):887-893. doi: 10.1016/j.pan.2022.07.008. Epub 2022 Jul 19. PMID: 35872074.
- Guo YY, Li HX, Zhang Y, He WH. Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discov Med. 2019 Feb;27(147):101-109. PMID: 30939294.
- Kiss L, Fűr G, Pisipati S, Rajalingamgari P, Ewald N, Singh V, Rakonczay Z Jr. Mechanisms linking hypertriglyceridemia to acute pancreatitis. Acta Physiol (Oxf). 2023 Mar;237(3):e13916. doi: 10.1111/apha.13916. Epub 2023 Jan 13. PMID: 36599412.
- Yang HY, Liang ZH, Xie JL, Wu Q, Qin YY, et al. Gelsolin impairs barrier function in pancreatic ductal epithelial cells by actin filament depolymerization in hypertriglyceridemia-induced pancreatitis in vitro. Exp Ther Med. 2022 Apr;23(4):290. doi: 10.3892/etm.2022.11219. Epub 2022 Feb 16. PMID: 35317441.
- Chang YT, Chang MC, Tung CC, Wei SC, Wong JM. Distinctive roles of unsaturated and saturated fatty acids in hyperlipidemic pancreatitis. World J Gastroenterol. 2015 Aug 28;21(32):9534-43. doi: 10.3748/wjg.v21.i32.9534. PMID: 26327761.
- Carta G, Murru E, Banni S, Manca C. Palmitic Acid: Physio–logical Role, Metabolism and Nutritional Implications. Front Physiol. 2017 Nov 8;8:902. doi: 10.3389/fphys.2017.00902. eCollection 2017. PMID: 29167646.
- Ben-Dror K, Birk R. Oleic acid ameliorates palmitic acid-induced ER stress and inflammation markers in naive and cerulein-treated exocrine pancreas cells. Biosci Rep. 2019 May 14;39(5):BSR20190054. doi: 10.1042/BSR20190054. Print 2019 May 31. PMID: 30992393.
- Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019 Nov;68(11):915-932. doi: 10.1007/s00011-019-01273-5. Epub 2019 Jul 30. PMID: 31363792.
- Criddle DN, Raraty MG, Neoptolemos JP, Tepikin AV, Peter–sen OH, Sutton R. Ethanol toxicity in pancreatic acinar cells: mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci USA. 2004 Jul 20;101(29):10738-43. doi: 10.1073/pnas.0403431101. Epub 2004 Jul 9. PMID: 15247419.
- Maléth J, Balázs A, Pallagi P, Balla Z, Kui B, Katona M, et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology. 2015 Feb;148(2):427-39.e16. doi: 10.1053/j.gastro.2014.11.002. Epub 2014 Nov 7. PMID: 25447846.
- Gezginci-Oktayoglu S, Sancar S, Karatug-Kacar A, Bol–kent S. miR-375 induces adipogenesis through targeting Erk1 in pancreatic duct cells under the influence of sodium palmitate. J Cell Phy–siol. 2021 May;236(5):3881-3895. doi: 10.1002/jcp.30129. Epub 2020 Oct 26. PMID: 33107061.
- Noel P, Patel K, Durgampudi C, Trivedi RN, de Oliveira C, Crowell MD, et al. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut. 2016 Jan;65(1):100-11. doi: 10.1136/gutjnl-2014-308043. Epub 2014 Dec 10. PMID: 25500204.
- Khatua B, Yaron JR, El-Kurdi B, Kostenko S, Papachristou GI, Singh VP. Ringer’s Lactate Prevents Early Organ Failure by Providing Extracellular Calcium. J Clin Med. 2020 Jan 18;9(1):263. doi: 10.3390/jcm9010263. PMID: 31963691.