Introduction
Reduction in vitamin D level in the body is viewed as a potential risk factor for arterial hypertension (AH) [1, 2], low level of 25(OH)D is associated with increased level of blood pressure (BP) [2–6] and increased incidence of AH [7]. According to the European guidelines [1], individuals with cardiovascular diseases, AH in particular, belong to the risk group for whom it is recommended to determine the level of 25(OH)D since untreated vitamin D deficiency can significantly reduce efficiency of the background therapy or change the diseasecourse. At present, the findings of more than 50 studies including supplementary intake of various vitamin D preparations (D2 (ergocalciferol), D3 (cholecalciferol), calcitriol (1.25-hydroxivitamin D3) etc.) with assessment of their effect on the values of systolic (SBP) and diastolic blood pressure (DBP) [8] are available. Duration of vitamin D intake in these studies varied from 2 weeks to 12 months. In all studies the doses of vitamin D higher than 600 IU a day (from 800 tо 8,571 IU per day) were used. The highest effect on lowering SBP was described in a placebo-controlled study [9] in patients with diabetes mellitus type 2. According to that study, single intake of ergocalciferol at a dose of 100,000 IU demonstrated a 14 mm Hg reduction in SBP as compared to the placebo group.
Considering the fact that AH is a wide-spread disease and has an independent continuous relationship with the incidence of a number of cardiovascular events (stroke, myocardial infarction, sudden death, cardiac failure, end-stage renal disease), achieving the BP target level is one of the main tasks of the administered antihypertensive the–rapy [10, 11]. In view of modern data it is essential both to optimize the level of 25(OH)D in the body of people with AH and to choose maximum effective vitamin D dosages and regimens of intake when included into the complex therapy of AH.
Purpose of the study was to assess the effect of cholecalciferol intake at a daily dose of 2,000 IU on the serum level of 25(ОН)D total and BP against the background of antihypertensive therapy in people with AH stage II.
Materials and methods
We conducted a prospective single-center study of 115 individuals with AH stage II (91 females and 24 males), their mean age being 50.7 ± 7.1 years. The duration of follow-up period averaged 15.8 ± 1.8 months, from a minimum of 12 months to a maximum of 18 months. Verification of AH diagnosis, stage and risk was performed according to the European guidelines (2013) [11]. Research protocol was approved by the Committee for Biomedical Ethics of Grodno State Medical University. All subjects received full blood count, clinical urine examination, fas–ting blood sugar, serum urea, serum creatinine tests. Results in all cases were normal. Venous blood samples were taken after the overnight fast, 12–14 hours after the last intake of food and medications. All patients had electrocardiography, office systolic and diastolic blood pressure, and their anthropometric data were taken. During the overall follow-up period all patients were receiving antihypertensive therapy according to the European guidelines: either angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor antagonists (ARA) — losartan, or diuretics (hydrochlorothiazide or indapamide) as a part of combination therapy, or calcium antagonists (CA) – amlodipine, Beta-adrenergic blockers (β-blockers) or their combination.
Vitamin D status was assessed according to the serum level of vitamin D total (25(OH)D total = 25(ОН)D3 + 25(ОН)D2) using immunoenzymatic assay with original DRG reagents (Germany, Marburg) at the premi–ses of the Scientific Research Laboratory of Grodno State Medical University. We used singularly de-frozen plasma for analysis. According to the European guidelines [11], vitamin D level was considered optimal with 25(OH)D total 30–80 ng/ml, insufficient with 25(OH)D total 20–30 ng/ml, deficient with 25(OH)D total < 20 ng/ml.
25(OH)D total was assessed at baseline, at 3 months and at the end of the follow-up period. Every second patient on the alphabetical list, in addition to antihypertensive therapy, was recommended to take vitamin D in the form of cholecalciferol at a dose of 2,000 IU/d daily. After three months, the patients were invited by telephone to the polyclinic to have a 25-hydroxy vitamin D blood test taken, BP was also measured, and antihypertensive therapy was corrected when necessary. Seventy-two patients were receiving cholecalciferol for three months, 20 of these continued the intake of cholecalciferol for 6 months, 9 persons continued the intake for an average of 8.7 ± 2.1 months.
Statistical analysis was performed using «Statistica 10.0» (SN AXAR207F394425FA-Q). Data representation corresponded to the character of their distribution: in normal distribution (by Shapiro-Wilk test) — as a mean and standard deviation (M ± SD), in non-normal distribution — as a median (Me) and interquartile range [Q25-Q75].
To assess the association between the variables we used Spearman rank correlation analysis (R). To estimate the influence of several factors on the value of the studied index, we used ANOVA multivariate analysis of variance (Kruskal-Wallis test, Median test). Two dependent groups of the studied variables were compared according to the Wilcoxon test. When the number of groups was more than two, and for pair-wise comparison of significant diffe–rences between the groups, the Duncan’s test was used. The index «dynamics» (d) was calculated as a difference between values before and after administered therapy. The nil hypothesis was rejected at p ≤ 0.05.
Results
During the follow-up period, 38.8 % subjects were recei–ving monotherapy with antihypertensive drugs, 50.9 % were receiving combined therapy with two preparations, 6.6 % were receiving three preparations. According to the group of antihypertensive drugs, the patients were divided into the following groups: 87.7 % subjects were receiving ACE inhibitors/ARA, 37.7 % were receiving thiazide or thiazide-like diuretics, 16 % were on CA, 18.9 % were on β-blockers.
At baseline, office SBP and DBP averaged 150 [140; 160]/90 [90; 100] mm Hg, heart rate — 73.3 ± 10.4 beats/min, height 166.6 ± 8.6 сm, weight 85.9 ± 17.5 kg, body mass index (BMI) 30.9 ± 5.8 kg/m2.
Over 15.8 ± 1.8 months of follow-up there was a significant (p < 0,0001) reduction in both office SBP and DBP, their means averaged 130 [125; 140] and 80 [80; 90] mm Hg correspondingly, while the HR did not change (p = 0.37) and was 71.7 ± 13.3 beats/min. Target levels of BP as for the results of office measurements were achieved in 83.9 % and 87.1 % cases for SBP and DBP levels correspondingly.
Serum 25(ОН)D total in the whole group of the studi–ed patients at baseline was 24.8 [17.02; 34.06] ng/ml, at the end of the follow-up period — 41.7 [33.1; 53.5] ng/ml (p = 0.00001).
/22-1.jpg)
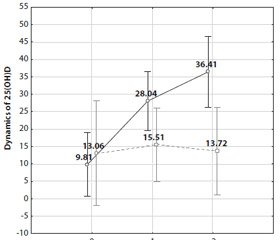
We also analyzed the effect of certain groups of antihypertensive drugs on the dynamics of serum 25(ОН)D total and its level at the end of the follow-up. It was established that administration of diuretics (hydrochlorothiazide at a dose of 12.5 mg and higher, or indapamide at a dose of 1.5 mg and higher) influenced the dynamics of serum 25(ОН)D (F = 5.35; р = 0.02) and its level (F = 11.8; р = 0.0009). As shown in Figure 1, the highest 25(ОН)D dynamics was in the group of those patients who were not receiving diure–tics at the background of long-term (6 months and longer) ingestion of cholecalciferol (36.4 [25.1; 47.7], p < 0.05) as compared to the values of the dynamics in all groups apart from one group consisting of individuals who received cholecalciferol for 3 months but did not receive diuretics either. In the group of patients, who received thiazide/thiazide-like diuretics, serum 25(ОН)D dynamics after a year of the follow-up made up 13.7 [8.9; 19.9], which was lower (p < 0.05) than in the group without intake of diuretics. Serum 25(ОН)D dynamics as well as its level at the end of the follow-up were not significantly influenced by the intake of other groups of antihypertensive drugs (ACE inhibitors, ARA, β-blockers, CA).
Taking into consideration the data described above, all the studied patients with AH stage II were divided into the following groups:
— group 0 — those receiving neither a diuretic as a part of combination antihypertensive therapy, nor cholecalci–ferol for correction of vitamin D level in the body;
— group 1 — those not receiving a diuretic but taking cholecalciferol at 2,000 IU/d;
— group 2 — those not receiving cholecalciferol, but taking a diuretic as a part of combination antihypertensive therapy;
— group 3 — those taking both a diuretic and cholecalciferol at 2,000 IU/d.
Medians and interquartile range of the studied indices and their dynamics are shown in Table 1. Comparison of the above groups according to the intake of other preparation groups (ACEi/ARA, CA, β-blockers) as a part of combination antihypertensive therapy did not reveal any significant differences (Kruskal-Wallis test). The groups were also matched (p > 0.05) for age, BMI, DBP level. SBP level was significantly higher in group 3 as compared to group 0 and group 1 (see Table 1).
/23-1.jpg)
At baseline, the groups did not differ in terms of serum 25(ОН)D total level (p > 0.05 in all cases). Following the therapy, optimal serum 25(ОН)D total level was observed in 82.3 % subjects of group 0, in 92% of group 1, in 72.7 % of group 2, and in 79.3 % of group 3, while the duration of cholecalciferol intake was comparable (p > 0.05) and amounted to 4.5 ± 2.3 months in group 1, 4.4 ± 2.2 months in group 3. The highest average level of serum 25(ОН)D total after the therapy was observed in group 1 (see Table 1) and the comparison of the above groups (Kruskal-Wallis test) showed significant diffe–rences between group 1 and group 0 (р = 0.04), group 2 (р = 0.02), group 3 (р = 0.002). In group 1 the dynamics of 25(ОН)D total was maximal — 32.3 [18.8; 49.6] and significantly higher than in group 0 (р = 0.0009), group 2 (р = 0.05) and group 3 (р = 0.02).
/24-1.jpg)
At the end of the follow-up, the groups did not differ according to SBP, DBP levels and DBP dynamics (p > 0.05 in all cases); however there were differences in SBP dyna–mics. As indicated in Table 1 and Figure 2, SBP dynamics was maximal (–27.4 ± 17.9) in group 3 and, as revealed by the analysis of variance (Duncan’s test), it was significantly different from all groups: from group 2 (–17.3 ± 14.9; р = 0.04); from group 1 (–9.6 ± 14.7; р = 0.0007) and from group 0 (–8.7 ± 16.1; р = 0.0005). Similar findings were obtained when using other methods of statistical analysis for comparing dSBP between the groups by means of Median test (Chi2 = 8.99; р = 0.0295) and by Kruskal-Wallis test (Н = 19.08397; p= 0.0003).
In all subjects with AH stage II, we established a significant correlation between intake of a diuretic and SBP dynamics (R = –0.41; р = 0.000017). We established a significant influence of cholecalciferol administration on SBP dynamics (F = 4.1; р = 0.046), as well as a significant influence of diuretic intake on SBP dynamics (F = 14.3; р = 0.0003). Besides, in group 3 there was established a correlation between SBP dynamics and duration of cholecalciferol intake (R = 0.42; р = 0.023).
Having distributed the examined subjects with AH into subgroups according to the intake of ACEi/ARA, CA, β-blockers with or without cholecalciferol intake, we did not obtain similar convincing and significant findings concerning the influence on either SBP or DBP as during concomitant use of a diuretic and cholecalciferol.
Discussion
Over the recent 5 years, there have been published seve–ral meta-analyses of randomized, placebo-controlled stu–dies evidencing BP reduction with supplementary intake of vitamin D [8, 12, 14]. One of the first meta-analyses de–monstrated a significant reduction in SBP (–6.2 mm Hg, 95% CI from –12.32 to –0,04) and DBP (–3.1 mm Hg, 95% CI from –5.5 to –0.6) in subjects with AH but not in normotensive patients as compared to placebo [12]. The meta-analysis performed in 2014 [13] showed a significant reduction only in DBP (–1.31, 95% CI –2.28, –0.34 mm Hg, P = 0.01) for subjects with cardiovascular diseases. Data of the recent meta-analysis which included 46 studies (4,541 participants) did not demonstrate a significant reduction either in SBP or DBP in supplementary intake of vitamin D [8].
Simultaneously, there has been a continuous debate about what level of serum 25-hydroxivitamin D should be considered optimal, what doses and dosage frequency of vitamin D preparations are necessary to obtain the optimum. Although 25(ОН)D > 30 ng/ml is postulated to be optimal for health in general, there are no results indicating what level is necessary for obtaining maximal antihypertensive effect. The doses and forms of vitamin D preparations also vary greatly in the studies. In the recent meta-analysis [8], most studies used either small doses of vitamin D preparations or intermittent dosage regimens (daily, monthly or less often). Intermittent dosage regimens can have different biological effects as compared to small regular doses. Moreover, results of meta-analysis make it difficult to differentiate the role of low serum 25-hydroxivitamin D level from other important cardiovascular risk factors (age, obesity, smoking etc.) which also influence not only baseline level of vitamin D in the body but its dynamics as well.
Analysis of literature data revealed single studies which evaluated the results of concomitant use of antihypertensive drugs and vitamin D but we did not find any publications where diuretics were referred to as an antihypertensive group. In our study, the patients were not on monotherapy with diuretics either, the latter were used as a part of combination therapy with ACEi or ARA. More often, results of the studies referred to the fact of using antihypertensive drugs in a certain percentage of cases or in connection with group of patients who received ACEi. However, absence of significant associations between 25(ОН)D level and BP in subjects receiving ACEi, does not rule out the possibility of such associations. Due to the effect of ACEi on rennin-angiotensin-aldosterone system, it is impossible to establish additional hypotensive effect of vitamin D, whose influence on BP is, first and foremost, explained by its ability to suppress rennin and angiotensin II secretion [14–17]. Moreover, it has been established that suppression of rennin secretion by vitamin D through activation of its receptors occurs irrespective of calcium and parathyroid hormone homeostasis and changes of water-electrolyte exchange [15].
Of interest are the results of double blind placebo-controlled study of people with AH stage I–II in which CA nifedipine at a dose of 30 mg/d was used as a part of antihypertensive therapy and the participants were divided into two groups with supplementary intake of vitamin D (n = 63, 2,000 IU/d) or placebo (n = 63). Ambulatory monitoring of BP was performed three times — at baseline, after 3 and 6 months of the follow-up. In the group of patients who received vitamin D there was a significant increase in serum 25(ОН)D level (from 19.4 ± 11.6 ng/ml tо 34.1 ± 12.2 ng/ml at 6 months, р < 0.001). Furthermore, in this group as compared to the placebo, after 6 months there was a significant (р < 0.001) reduction observed in SBP by 6.2 mm Hg and in DBP by 4.2 mm Hg. The researchers came to the conclusion that administration of vitamin D preparations resulted in BP reduction and could be used as adjuvant therapy for patients with AH stage I–II [18].
We are also aware of interesting results from a prospective randomized placebo-controlled study [19] of 283 healthy individuals who were divided into four groups: those ta–king placebo, cholecalciferol at a dose of 1,000, 2,000 and 4,000 IU/d respectively. BP was measured in all subjects at baseline, after 3 and 6 months. There were no changes in DBP values, while SBP values (p = 0.04) changed significantly during the follow-up. At 3 months in group 1, increase in SBP by 1.7 mm Hg was observed, while in group 2, SBP decreased by 0.66 mm Hg, and in groups 3 and 4 — by 3.4 and 4.0 mm Hg respectively. The authors made the following conclusion from the data received: the higher the dose of cholecalciferol, the lower SBP values. Moreover, BP values had a significant reverse correlation with serum 25(OH)D level.
Thus, current literature data concerning efficiency of vitamin D supplementary intake by people with AH or by healthy individuals are ambivalent and even contradictory.
Conclusions
1. Supplementary intake of cholecalciferol at a dose of 2,000 IU/d in people with AH stage II at the background of antihypertensive therapy for 4.4 ± 2.2 months without a diuretic allows to optimize vitamin D level in 92 % cases while with a diuretic — in 79 % cases. Thiazide/thiazide-like diuretics negatively influence the increase of serum 25(OH)D
total level. The intake of other groups of antihypertensive drugs — ACEi, ARA, CA, β-blockers — does not influence significantly the dynamics of 25(OH)D total.
2. Duration of cholecalciferol intake is directly correlated with SBP dynamics.
3. Concomitant use of a diuretic (hydrochlorothiazide at a dose of 12.5 mg or indapamide 1.5 mg) and cholecalcife–rol at a dose of 2,000 IU/d in therapy of AH stage II allows to obtain the highest hypotensive effect on SBP values without the risk of vitamin D overdosing, and thus should be used in clinical practice.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Płudowski P, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe — recommended vitamin D intakes in the ge–neral population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64(4):319-27. PMID: 24002961.
2. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007 Jul;20(7):713-9. doi: 10.1016/j.amjhyper.2007.01.017.
3. Forouhi NG, et al. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective study 1990-2000. Diabetes. 2008 Oct;57(10):2619-25. doi: 10.2337/db08-0593.
4. Smotkin-Tangorra M, et al. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab. 2007;20:817-23. PMID: 17849744.
5. Gannage-Yared MH, et al. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol. 2009;160:965-71. doi: 10.1530/EJE-08-0952.
6. Snezhitskiy VA, Yankovskaya LV, Povorozniuk VV, et al. Vitamin D deficiency/insufficiency among residents of the Western Region of Belarus suffering from cardiovascular pathology. Standardy Medyczne. Pediatria. 2012;5:577-82.
7. Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283 537 participants. Eur J Epidemiol. 2013;28(3):205-21. doi: 10.1007/s10654-013-9790-2.
8. Beveridge LA. Effect of Vitamin D Supplementation on Blood Pressure A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA Intern Med. 2015 May;175(5):745-54. doi: 10.1001/jamainternmed.2015.0237.
9. Sugden JA, Davies JI, Witham MD, et al. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008 May;25(3):320-5. doi: 10.1111/j.1464-5491.2007.02360.x.
10. Grassi G, Cifkova R, Laurent S, et al. Blood pressure control and cardiovascular risk profile in hypertensive patients from central and eastern European countries: results of the BP-CARE study. European Heart Journal. 2011;32:218-25. doi: 10.1093/eurheartj/ehq394.
11. Mancia G. ESH/ESC Guidelines for the management of arterial hypertension 2013. Journal of Hypertension. 2013 July;31(7):1281-1357. doi: 10.1097/01.hjh.0000431740.32696.cc.
12. Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009 Oct;27(10):1948-54. doi: 10.1097/HJH.0b013e32832f075b.
13. Kunutsor SK. Vitamin D and high blood pressure: causal association or epiphenomenon? Eur J Epidemiol. 2014;29:1-14. doi: 10.1007/s10654-013-9874-z.
14. Li Y.C. Vitamin D: a negative endocrine regulator of the reninangiotensin system and blood pressure. J Ster Biochem Molec Biol. 2004 May;89-90(1-5):387-92. doi: 10.1016/j.jsbmb.2004.03.004.
15. Kong J. Targeted vitamin D receptor expression in juxtaglomerular cells supresses rennin expression independent of parathyroid hormone and calcium. Kidney International. 2008 Dec;74(12):1577-81. doi: 10.1038/ki.2008.452.
16. Kezhun LV, Jankovskaja LV. Plasma renin activity, endothelial function, and vitamin d status in women with hypertension in premenopausal and early postmenopausal periods. Zhurnal Grodnenskogo Gosudarstvennogo Medicinskogo Universiteta. 2014;4(48):37-42.
17. Kezhun LV, Jankovskaja LV. Role of renin-angiotensin-aldosterone system and vitamin d in development of arterial hypertension in perimenopausal women. Zhurnal Grodnenskogo Gosudarstvennogo Medicinskogo Universiteta. 2013;1(41):14-7.
18. Chen WR, Liyu ZY, et al. Vitamin D and nifedipine in the treatment of Chinese patients with grades I–II essential hypertension: A randomized placebo-controlled trial. Atherosclerosis. 2014;235(1):102-9. doi: 10.1016/j.atherosclerosis.2014.04.011.
19. Forman JР. Plasma 25-hydroxy vitamin D le–vels and risk of incident hуреrtеnsiоn. Hypertension. 2007;49(5):1063-9. doi: 10.1161/HYPERTENSIONAHA.107.087288.

/22-1.jpg)
/23-1.jpg)
/24-1.jpg)