Резюме
Актуальність. Дефіцит вітаміну D поширений в усіх країнах світу. Недавні дослідження показали зв’язок між дефіцитом вітаміну D та захворюваннями кістково-м’язової системи. Метою даного дослідження є вивчення частоти дефіциту та недостатності вітаміну D у пацієнтів різного віку із захворюваннями кістково-м’язової системи, а також вплив на зазначені показники сезонності. Матеріали та методи. Обстежено 3460 пацієнтів Українського науково-медичного центру проблем остеопорозу віком від 1 до 92 років, які були направлені лікарями різного профілю з метою оцінки стану кісткової тканини. Переважну більшість становили хворі із системним остеопорозом та його ускладненнями, остеохондрозом хребта, остеоартрозом колінних та кульшових суглобів (середній вік — 52,9 ± 21,1 року). Переважну більшість становили жінки — 83,5 %. Дослідження 25(ОН)D та паратгормона проводили за допомогою електрохемілюмінесцентного методу на аналізаторі Elecsys 2010 (Roche Diagnostics, Німеччина) тест-системами cobas. При аналізі використовували пакети програм Statistica 6.0 Copyright StatSoft, Inc. 1984–2001. Результати. Серед хворих із патологією кістково-м’язової системи найвищий рівень 25(ОН)D спостерігається у віковій групі 1–9 років, найнижчий — у віковій групі 80 років та старше. Вік вірогідно впливає на рівень 25(ОН)D. Частота дефіциту вітаміну D серед обстежених із патологією кістково-м’язової системи становила 37,3 %, недостатності вітаміну D — 30,6 %, нормальні рівні показника вітаміну D спостерігали в 32,1 % випадків. Нормальний рівень вітаміну D відмічався в 38,0 % дітей, 33,2 % дорослих, 29,6 % пацієнтів літнього віку. Місяць, в якому проводилось дослідження, вірогідно впливає на рівень 25(ОН)D (F = 7,49; p < 0,0000001). Найбільші вірогідні відмінності рівня 25(ОН)D у літній період порівняно із зимовим спостерігали у вікових групах 10–19 років (18,2 %), 40–49 років (17,3 %), 30–39 років (16,2 %) та 1–9 років (16,1 %). Не відмічали відмінностей показника залежно від сезону у хворих літнього віку (60 років й старше) із патологією кістково-м’язової системи. Висновки. Незважаючи на додатковий прийом комбінованих препаратів кальцію й вітаміну D більшістю пацієнтів із патологією кістково-м’язової системи, тільки у 37,9 % дітей, 33,2 % дорослих та 29,6 % хворих літнього віку спостерігали нормальні рівні 25(ОН)D у сироватці крові, що диктує необхідність обов’язкового скринінгового визначення рівня вітаміну D у пацієнтів із захворюваннями кістково-м’язової системи та додаткового призначення вітаміну D у клінічній практиці (рекомендації для країн Центральної та Східної Європи).
Актуальность. Дефицит витамина D распространен во всех странах мира. Недавние исследования показали связь между дефицитом витамина D и заболеваниями костно-мышечной системы. Целью данного исследования является изучение частоты дефицита и недостаточности витамина D у пациентов разного возраста с заболеваниями костно-мышечной системы, а также влияние на эти показатели сезонности. Материалы и методы. Обследовано 3460 пациентов Украинского научно-медицинского центра проблем остеопороза в возрасте от 1 до 92 лет, направленных врачами разного профиля с целью оценки состояния костной ткани. Преобладающее большинство составили больные с системным остеопорозом и его осложнениями, остеохондрозом позвоночника, остеоартрозом коленных и тазобедренных суставов (средний возраст — 52,9 ± 21,1 года). Подавляющее большинство составили женщины — 83,5 %. Исследование 25(ОН)D и паратгормона проводили с помощью электрохемилюминесцентного метода на анализаторе Elecsys 2010 (Roche Diagnostics, Германия) тест-системами cobas. При анализе использовали пакеты программ Statistica 6.0 Copyright StatSoft, Inc. 1984–2001. Результаты. Среди больных с патологией костно-мышечной системы наиболее высокий уровень 25(ОН)D наблюдается в возрастной группе 1–9 лет, самый низкий — в возрастной группе 80 лет и старше. Возраст достоверно влияет на уровень 25(ОН)D. Частота дефицита витамина D среди обследованных с патологией костно-мышечной системы составила 37,3 %, недостаточности витамина D — 30,6 %, нормальный уровень витамина D наблюдали в 32,1 % случаев. Нормальный уровень витамина D отмечался у 38,0 % детей, 33,2 % взрослых, 29,6% пациентов пожилого возраста. Месяц, в котором проводилось исследование, достоверно влияет на уровень 25(ОН)D (F = 7,49; p < 0,0000001). Наиболее достоверные различия уровня 25(ОН)D в летний период по сравнению с зимним наблюдали в возрастных группах 10–19 лет (18,2 %), 40–49 лет (17,3 %), 30–39 лет (16,2 %) и 1–9 лет (16,1 %). Не отмечали различий показателя в зависимости от сезона у больных пожилого возраста (60 лет и старше) с патологией костно-мышечной системы. Выводы. Несмотря на дополнительный прием комбинированных препаратов кальция и витамина D большинством пациентов с патологией костно-мышечной системы, только у 37,9 % детей, 33,2 % взрослых и 29,6 % больных пожилого возраста наблюдали нормальные уровни 25(ОН)D в сыворотке крови, что диктует необходимость обязательного скринингового определения уровня витамина D у пациентов с заболеваниями костно-мышечной системы и дополнительного назначения витамина D в клинической практике (рекомендации для стран Центральной и Восточной Европы).
Background. Vitamin D deficiency is prevalent in all the world countries. Recent studies show the correlation between vitamin D deficiency and musculoskeletal disorders. The purpose of this study is to examine vitamin D deficiency and insufficiency prevalence in patients of various ages, who have musculoskeletal disorders, and to reveal the influence of seasonal factors on these conditions. Materials and methods. 3460 patients of the Ukrainian Scientific Medical Center of Osteoporosis Problems, aged 1 to 92 years, who were referred by other specialists to the center for bone state evaluation, were examined. A majority of the patients presented with osteoporosis and its complications, spinal osteochondrosis, knee and hip osteoarthritis (mean age — 52.90 ± 21.10 years). Most of the patients were women (83.5 %). 25(ОН)D and parathyroid hormone analyses were performed by means of electrochemiluminescent method (Elecsys 2010 analyzer, Roche Diagnostics, Germany) and cobas test-systems. Statistica 6.0 software package (Copyright StatSoft, Inc., 1984–2001) was also used. Results. Among the patients with musculoskeletal pathology, the highest 25(ОН)D level was noted in the age group of 1–9 years and the lowest — in the age group of 80 and over. Age negatively influenced 25(ОН)D values. Prevalence of vitamin D deficiency among the patients with musculoskeletal pathology was 37.3 %, vitamin D insufficiency — 30.6 %; 32.1 % of patients had normal vitamin D status. Normal 25(OH)D level was found in 38.0 % of children, 33.2 % of adults and in 29.6 % of elderly patients. Month of blood sampling had a significant influence on 25(ОН)D content (F = 7.49; p < 0.001). The highest significant differences in 25(ОН)D levels during the summer vs. winter months were observed in the age groups of 10–19 (18.2 %), 40–49 (17.3 %), 30–39 (16.2 %) и 1–9 years (16.1 %). There were no significant seasonal differences observed in the elderly patients (60 years and older) with musculoskeletal pathology. Conclusions. Despite the combined calcium and vitamin D supplementation in most patients with musculoskeletal pathology, only 37.9 % of children, 33.2 % of adults and 29.6 % of the elderly people had normal 25(ОН)D values and thus required screening examination of vitamin D level in patients with musculoskeletal disorders and additional vitamin D prescription (Guidelines for the Central and Eastern Europe).
Introduction
Vitamin D deficiency is prevalent in all countries around the world [1–3]. According to the recent evaluations, in the US, Canada and Europe from 20 to 100 % of elderly men and women who are private home residents, rather than nursing home residents, have a vitamin D deficiency. Risk of a vitamin D deficiency and insufficiency is equally high among the children and adults of young and middle age all over the world [4].
Vitamin D is known as a fat-soluble vitamin, which promotes an effective absorption of dietary calcium and phosphorus, and as such, has vital importance for growth and bone mineralization. Vitamin D is synthesized in the human skin under the influence of solar ultraviolet radiation and is the major source of vitamin D for most children and adults [5, 6]. Skeletal disorders associated with vitamin D deficiency include rickets with associated bone deformities, osteoporosis and its complications and non-specific pain syndromes associated with osteomalacia [5]. Studies show the presence of vitamin D receptors in skeletal muscular nuclei, signifying vitamin D’s connection with skeletal muscles function [7]. A study by M. Çidem et al. involving 14 925 patients aged 20–99 years with bone and muscular disorders observed vitamin D deficiency in 73.9 % of them [8]. Serum levels of 25(ОН)D were affected by season, age and sex.
Vitamin D deficiency is also associated with cardiovascular diseases, diabetes mellitus, cancer, infections, autoimmune diseases [5, 9–12]. In the European League Against Rheumatism (EULAR)-initiated study, 625 patients with rheumatoid arthritis (mean age — 55 ± 11 years) recruited from 13 European countries were found to have a significantly lower mean 25(ОН)D serum level, amounting to 17.6 ± 9.8 ng/ml in winter months [13].
Our previously conducted studies revealed a great number of Ukrainians suffering from vitamin D deficiency and insufficiency, equally affected by age, sex, body mass index, region of residence and season [14–18].
The purpose of this study is to examine vitamin D deficiency and insufficiency prevalence in patients of various ages who have bone and muscular disorders, and to reveal the influence of seasonal factors on these conditions.
Materials and methods
3460 patients of the Ukrainian Scientific-Medical Center of Osteoporosis Problems, aged 1 to 92 years, who were referred by other specialists to the Center for a musculoskeletal evaluation. A majority of the patients presented with osteoporosis and its complications, spinal osteochondrosis and, knee and hip osteoarthritis (mean age — 52.9 ± 21.1 years). Most of the patients were women (83.5 %), their mean age being 55.6 ± 19.4 years, while the men’s mean age was 38.6 ± 17.3 years (р < 0.001).
Patients’ distribution as to age and sex is shown in table 1.
Patients were further divided into three age groups: children (1–18 years), adults (19–59 years), elderly people (60 years and older) (table 2).
All of the subjects were evaluated as to the total level of 25(ОН)D (25(ОН)D total) and parathyroid hormone (PTH) in serum. 25(ОН)D total represents dietary and sun induced cutaneous vitamin D and is a measure of a person’s vitamin D status [5].
25(ОН)D and PTH analyses were performed by means of electrochemiluminescent method (Elecsys 2010 analyzer, Roche Diagnostics, Germany) and cobas test-systems. This method is, to date, considered the most sensitive, and is able to measure the studied substance concentration on a broad scope and with a high precision.
Vitamin D status was evaluated according to the latest classification [7], vitamin D deficiency when serum levels of 25(ОН)D are lower than 20 ng/ml, vitamin D insufficiency when serum levels of 25(ОН)D are between 20 and 30 ng/ml. Serum levels of 25(ОН)D within the range of 31–100 ng/ml are considered normal. Levels above 150 ng/ml are considered to be potentially toxic.
Statistical analysis was performed by means of a descriptive statistics, Student criteria for unbound variables and one-way ANOVA dispersion analysis. Statistica 6.0 software package (Copyright StatSoft, Inc. 1984–2001) was also applied.
Results
Age and sex related to 25(ОН)D levels in patients with musculoskeletal pathology
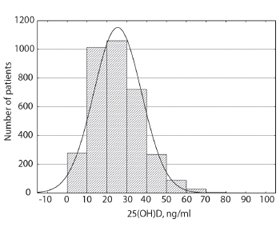
Histogram of patients’ 25(OH)D levels is presented in fig. 1.
Analysis of 25(ОН)D concentration in all the age groups (table 3) showed the highest levels in the age group of 1–9 years (30.6 ± 15.1 ng/ml), while the lowest levels were is found in the age group of 80 and over (20.4 ± 11.4 ng/ml). Mean level of the 25(ОН)D was 26.2 ± 11.9 ng/ml. According to one-way ANOVA analysis, the age significantly influenced 25(ОН)D values (F = 11,7; p < 0,000001).
We didn’t find any significant difference in 25(ОН)D levels in children (boys (n = 165) — 26.3 ± 14.3 ng/ml and girls (n = 222) — 27.2 ± 13.7 ng/ml, p = 0,54); in adults (men (n = 272) — 25.9 ± 12.5 ng/ml and women (n = 1185) — 26.0 ± 11.6 ng/ml, p = 0.87); in the elderly people (men (n = 134) — 24.2 ± 12.0 ng/ml and women (n = 1482) — 24.1 ± 11.6 ng/ml, p = 0.98).
We found that the prevalence of deficiency and insufficiency in patients with musculoskeletal complaints was 37.3 and 30.6 % respectively (fig. 2).
34.8 % of children, 36.8 % of adults and 39.2 % of the elderly people were found to be vitamin D deficient and an additional 27.1 % of children, 31.0 % of adults and 31.1 % of the elderly people were vitamin D insufficient. Only 38.0 % (children), 33.2 % (adults), 29.6 % (elderly people) were vitamin D sufficient.
25(ОН)D level and its relation to age in patients with musculoskeletal disorders
Correlation and regression analyses of relation between 25(ОН)D levels and age are shown in fig. 3 and table 4.
The highest correlation coefficient between the 25(ОН)D levels and age was observed in children (r = –0.34; p < 0.001). Vitamin D status diminished with advancing age. The group of adults (r = 0.09; p < 0.001) and elderly people (r = –0.12; p < 0.001) had different vectors for 25(ОН)D levels changing with age; diminishing in the elderly, but slightly increasing in the younger adults.
Influence of age and the month of the year on 25(ОН)D levels of patients with musculoskeletal pathology
One-way ANOVA dispersion analysis revealed a significant influence on 25(ОН)D levels (F = 7.49: p < 0.001). The highest levels were observed in the months of August (28.6 ± 11.4 ng/ml) and September (28.6 ± 11.6 ng/ml) and the lowest in the months of February (23.1 ± 11.6 ng/ml) and March (23.4 ± 11.3 ng/ml) (fig. 4).
The age of some of the patients had a significant influence on the seasonal variation in 25(ОН)D levels: 10–19 years (F = 3.79; p = 0.000056), 20–29 years (F = 2.97; p = 0.0013), 30–39 years (F = 2.84; p = 0.0017), 40–49 years (F = 2.22; p = 0.014), 50–59 years (F = 2.22; p = 0.017) and 60–69 years (F = 3.25; p = 0.00023). There was no significant influence on the seasonal variation in 25(OH)D levels in children up to 9 years and patients of 70 years and older.
Children had the lowest 25(ОН)D levels in February (20.8 ± 10.9 ng/ml), and the highest in August (35.7 ± 11.1 ng/ml); adults had the lowest 25(ОН)D levels in February (23.5 ± 12.0 ng/ml) and March (23.1 ± 10.4 ng/ml), and the highest in August (30.4 ± 10.8 ng/ml) and September (29.9 ± 11.1 ng/ml); elderly patients had the lowest 25(ОН)D levels in February (23.1 ± 11.3 ng/ml) and March (23.3 ± 11.0 ng/ml) and the highest in September (26.7 ± 11.1 ng/ml) (fig. 5).
Age and seasonal influence on 25(ОН)D level of patients with musculoskeletal pathology
Patients were further divided into two groups depending on season: A — serum 25(OH)D was determined in Nov 1 to April 30 (winter); B — May 1 to Oct 31 (summer) (table 6). The greatest difference in values for the summer period contrasted with the winter period was observed in the age groups of 10–19 years (18.2 %), 40–49 years (17.3 %), 30–39 years (16.2 %) and 1–9 years (16.1 %). No differences in serum 25(ОН)D levels throughout the year in the elderly patients (60 years and older).
Discussion
Vitamin D deficiency and insufficiency on chronic disease progression is a topical issue for scientists and medical practitioners around the world. The number of publications on the topic is ever increasing, as well as the number of international guidelines on vitamin D deficiency and insufficiency diagnostics, prophylaxis and treatment [19–22].
Before 2016, cross-sectional epidemiological studies performed in Ukraine [1, 15–17] showed a high incidence of vitamin D deficiency and insufficiency among the local population (90–95 %). We’ve also used several international studies for reference and comparison, among them MORE (International Multiple Outcomes of Raloxifene Evaluation Study) held in 25 countries in women older than 65 years who had osteoporosis [23]. In line with the MORE study we observed a seasonal influence on 25(ОН)D levels. The mean level of 25(ОН)D was 55.2 nmol/l (22.1 ng/ml) and higher than in our previous study of female patients [1, 14]. However the 25(ОН)D levels were lower in the patients with musculoskeletal pathology (24.2 ng/ml for the patients 60 years and older vs. 22.1 ng/ml). All of the patients in the MORE study were taking calcium and vitamin D supplements before the laboratory serum analysis like most patients in our own study. Nevertheless, we didn’t include similar patients into the cross-sectional epidemiological study.
The large-scale study of vitamin D deficiency (Survey in Europe on Nutrition and the Elderly: a Concerted Action (SENECA)) [24] examined blood samples taken exclusively during the winter months, and only in patients of over 65 years who didn’t take any vitamin D supplements. The mean 25(ОН)D level in this study was 33 nmol/l (13.2 ng/ml) while in our patients taking part in a cross-sectional epidemiological study it was 30.4 nmol/l which is similar to the European population. However, our patients with musculoskeletal disorders had a higher 25(ОН)D level (24.4 ng/ml in patients of over 60 years in winter months vs.13.2 ng/ml).
Our study on 25(ОН)D level is the first study in the Ukraine involving patients of the Ukrainian Center of Osteoporosis who had musculoskeletal disorders. These patients were under doctor’s supervision and were prescribed calcium (800–1000 mg per day) and vitamin D (400–800 IU per day) supplements, and adjusted their diet to include higher intakes of calcium and vitamin D. 63 % patients 50 years and older used the calcium and vitamin D supplementations unregularly. Both methods might have been responsible for higher circulating levels of 25(OH)D.
Our findings show that 68.0 % of patients with musculoskeletal disorders were vitamin D deficient or insufficient 30.6 % of which were deficient or insufficient. A Danish study involving patients with spinal osteochondrosis reported a similar incidents which 33 % were vitamin D deficient [25].
Similarly, our findings correspond to the ones by Panwar A. et al. In this study, patients with musculoskeletal disorders had significant vitamin D deficiency with prevalence varying from 50.8 to 53.6 % during the winter months with the mean 25(ОН)D level of 21.4 ± 13.2 ng/ml [26].
An Egyptian study of patients with fibromyalgia revealed a significantly lower mean 25(ОН)D level (15.1 ± 6.1 ng/ml) compared to our data (25.2 ± 12.0 ng/ml) [27].
B. Heidari et al. examined patients with a non-specific skeletal pain and found that 63.4 % were vitamin D deficient [28].
A large-scale study involving 14 925 patients 73.9 % with musculoskeletal disorders, aged 20–99 years were found to be vitamin D deficient [29]. The authors of this study emphasized the seasonal, age and sex influence on serum 25(ОН)D levels. In March, patients had the highest risk of vitamin D deficiency with the lowest serum 25(ОН)D levels. Our results show only age- and season-related influences, with 25(ОН)D’s lowest levels for adults in February (23.5 ± 12.0 ng/ml) and March (23.1 ± 10.4 ng/ml), for the elderly people (60–92 years) in February (23.1 ± 12.1 ng/ml) and March (23.3 ± 11.0 ng/ml).
The COMORA study involving 1413 patients with rheumatoid arthritis from 15 countries showed a mean serum 25(ОН)D levels of 27.3 ± 15.1 ng/ml. 8.5 % were vitamin D deficient and 54.6 % were vitamin D insufficient. In this study, vitamin D status was defined according to the Group for Research and Information on Osteoporosis (GRIO), as normal when vitamin D levels ≥ 30 ng/ml, insufficient when 10 ng/ml ≤ vitamin D levels < 30 ng/ml, and deficient when ≤ 10 ng/ml. The low incidence of vitamin D deficiency in this study was due to the fact that they 43 % of the patients were documented to have received vitamin D supplementation. Common factors associated with vitamin D deficiency were age, body mass index and patients’ level of education [10].
In the European League Against Rheumatism (EULAR)-initiated study, 625 patients with rheumatoid arthritis serum (mean age — 55 ± 11 years) recruited from 13 European countries were found to have a significantly lower mean serum 25(ОН)D levels of 17.6 ± 9.8 ng/ml in the winter months [13]. Mean serum 25(ОН)D levels were higher in all the age groups in our study (24.2 ± 13.8 in children; 24.5 ± 11.6 in adults and 24.4 ± 11.9 in the elderly patients).
Several studies have evaluated the effect of age on vitamin D status. In France, 68.3 % of elderly subjects (mean age — 82.0 ± 7.8 years) were vitamin D deficient and 24.1 % were insufficient [13]. 39.3 and 31.1 % of our patients over 60 years (mean age — 69.7 ± 6.9 years) were vitamin D deficient and insufficient respectively. The lower incidence is likely due to many of these patients receiving vitamin D and calcium supplementation by their treating physician.
34.9 and 38 % of the children who were examined at the Ukrainian Scientific-Medical Center of Osteoporosis were vitamin D deficient or insufficient respectively. A Korean study of children with non-specific leg pains revealed that 51.4 % were vitamin D deficient and 37.9 % were insufficient. The mean 25(ОН)D level of the Ukrainian children was 26.8 ± 14.0 ng/ml, while in the Korean study it was 17.2 ± 5.5 ng/ml [8]. 245 perimenopausal women-residents of China with systemic osteoporosis had an extremely low 25(ОН)D levels of 14.4 ± 6.6 ng/ml which was significantly lower than in our study [30].
Despite the combined calcium and vitamin D supplementation taken by most patients with a musculoskeletal pathology, only 37.9 % of children, 33.2 % of adults and 29.6 % of the elderly people had normal levels of 25(ОН)D and thus required additional vitamin D prescriptions (Guidelines for the Central and Eastern Europe).
Conclusions
The study allowed us to draw the following conclusions:
1. Among the patients with musculoskeletal pathology, the highest 25(ОН)D level was noted in the age group of 1–9 years (30,6 ± 15,1 ng/ml) and the lowest — in the age group of 80 and over (20.4 ± 11.4 ng/ml). Age negatively influenced 25(ОН)D level values.
2. Prevalence of vitamin D deficiency among the patients with musculoskeletal pathology was 37.3 %, vitamin D insufficiency — 30.6 %, normal vitamin D status – in 32.1 %. Normal 25(OH)D level was found in 38.0 % of children, 33.2 % of adults and in 29.6 % of elderly patients.
3. Month of blood-sampling had a significant influence on 25(ОН)D level values (F = 7.49; p < 0,0000001). The highest 25(ОН)D levels were observed in August (28.6 ± 11.4 ng/ml) and September (28.6 ± 11.6 ng/ml), the lowest — in February (23.1 ± 11.6 ng/ml) and March (23.4 ± 11.3 ng/ml).
4. Children had the lowest 25(ОН)D level in February (20.8 ± 10.9 ng/ml), and the highest in August (35.7 ± 11.1 ng/ml); adults had the lowest in February (23.5 ± 12.0 ng/ml) and in March (23.1 ± 10.4 ng/ml), and the highest in August (30.4 ± 10.8 ng/ml) and September (29.9 ± 11.1 ng/ml); elderly people had the lowest 25(ОН)D levels in February (23.1 ± 11.3 ng/ml) and in March (23.3 ± 11.0 ng/ml), and the highest in September (26.7 ± 11.1 ng/ml).
5. The highest significant differences in 25(ОН)D levels during the summer vs. winter months were observed in the age groups of 10–19 years (18.2 %), 40–49 years (17.3 %), 30–39 years (16.2 %) and 1–9 years (16.1 %). There were no significant seasonal differences observed in the elderly patients (60 years and older) with musculoskeletal pathology.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Povoroznyuk V, Pludowski P, Defitsyt i nedostatnistʹ vitaminu D: epidemiolohiya, diahnostyka, profilaktyka ta likuvannya [Vitamin D deficiency and deficiency: epidemiology, diagnosis, prevention and treatment]. Donetsk; 2014. 262 p. (In Ukrainian).
2. Pludowski P, Holick MF, Grant WB, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. 2017 (Feb)12. pii: S0960-0760(17)30031-6. doi: 10.1016/j.jsbmb.2017.01.021. [Epub ahead of print]
3. Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017 (Jun);18(2):153-165. doi: 10.1007/s11154-017-9424-1.
4. Hossein-nezhad A, Holick MF. Vitamin D for Health: A Global Perspective // Mayo Clin Proc. 2013.88(7):720–755. doi: http://dx.doi.org/10.1016/j.mayocp.2013.05.011.
5. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. DOI: 10.1056/NEJMra070553.
6. Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 2013 (Jan).5(1):51-108. doi: 10.4161/derm.24494.
7. Bischoff HA, Borchers M, Gudat F, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001 Jan;33(1):19-24.
8. Park MJ, et al. Prevalence of vitamin D deficiency in korean children presenting with nonspecific lower-extremity pain. Yonsei Med J. 2015 (Sep);56(5):1384-8. doi: 10.3349/ymj.2015.56.5.1384.
9. Povoroznyuk V, Synenky O, Balatska N, Płudowski P.Vitamin D status and disease activity in patients with rheumatoid arthritis. Post N Med. 2016;XXIX(10).705-708.
10. Hajjaj-Hassouni N, Mawani N, Allali F, et al. Evaluation of vitamin D Status in rheumatoid arthritis and its association with disease activity across 15 countries: «The COMORA Study». Int J Rheumatol. 2017;2017:5491676. doi: 10.1155/2017/5491676.
11. Annweiler C, Riou J, Alessandri A, et al. Clinical Identification of Geriatric Patients with Hypovitaminosis D: The ‘Vitamin D Status Predictor for Geriatrics’ Study. Nutrients. 2017 (Jun)27;9(7). pii: E658. doi: 10.3390/nu9070658.
12. Nakamura K, Nishiwaki T, Ueno K, et al. Age‑related decrease in serum 25‑hydroxyvitamin D concentrations in the frail elderly: a longitudinal study. J Bone Miner Metab. 2007;25:232-236. DOI: 10.1007/s00774-007-0755-y.
13. Vojinovic J, et al. European multicentre pilot survey to assess vitamin D status in rheumatoid arthritis patients and early development of a new Patient Reported Outcome questionnaire (D-PRO). Autoimmun Rev. 2017 May;16(5):548-554. doi: 10.1016/j.autrev.2017.03.002.
14. Povoroznyk V, Balatska N, Klymovytskyy F, et al. Rivenʹ vitaminu D u dorosloho naselennya riznykh rehioniv Ukrayiny [The level of vitamin D in adults of the different regions of Ukraine]. Problems of the Osteology. 2011;14(4).3-8. (In Ukrainian).
15. Povoroznyuk V, Balatska N, Muts V, et al. Vitamin D deficiency in Ukraine: a demographic and seasonal analysis. Gerontologija. 2012;13:4191-198.
16. Povoroznyuk V, Muts V, Balatska N. Vitamin D deficiency in elderly ukrainian population: impact of seasonal factors. Annals of gerontology and geriatric research. 2014;1(2):1009.
17. Povoroznyuk V, Pankiv I. Status vitaminu D u naselennya Bukovyny i Prykarpattya zalezhno vid mistsya prozhyvannya nad rivnem morya [The status of vitamin D in the population of Bukovina and Prykarpattya, depending on the place of residence above sea level]. Bol’, Sustavy, Pozvonochnik. 2016;2(22):7-10. (In Ukrainian).
18. Pludowski P, Grant WB, Bhattoa HP, et al. Vitamin D status in Central Europe. Int J Endocrinol. 2014;589587. doi: 10.1155/2014/589587.
19. Płudowski P, Karczmarewicz E, Bayer M, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe – recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64(4):319-27. PMID: 24002961.
20. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(7):1911-193. DOI: 10.1210/jc.2011-0385.
21. Al-Daghri NM, Al-Saleh Y., Aljohani Naji, et al. Vitamin D status correction in Saudi Arabia: an experts’ consensus under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO). Arch Oseoporosis. 2017. 2017;12(1):1. doi: 10.1007/s11657-016-0295-y.
22. Rizzoli R, Boonen S, Brandi ML, et al. Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin. 2013.29(4):305-313. doi: 10.1185/03007995.2013.766162.
23. Lips P, Duong T, Oleksik A, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifen evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212-1221. DOI: 10.1210/jcem.86.3.7327.
24. Lips P, Hosking D, Lippuner K, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245-254. DOI: 10.1111/j.1365-2796.2006.01685.x.
25. Johansen JV, Manniche C, Kjaer P. Vitamin D levels appear to be normal in Danish patients attending secondary care for low back pain and a weak positive correlation between serum level vitamin D and Modic changes was demonstrated: a cross-sectional cohort study of consecutive patients with non-specific low back pain. BMC Musculoskelet Disord. 2013 (Mar)4;14:78. doi: 10.1186/1471-2474-14-78.
26. Panwar A, Valupadas C, Veeramalla M, Vishwas HN. Prevalence of vitamin D deficiency in chronic and subacute low back pain patients in India: a triple-arm controlled study. Clin Rheumatol. 2017 (Aug)25. doi: 10.1007/s10067-017-3798-z. [Epub ahead of print]
27. Olama SM, Senna MK, Elarman MM, Elhawary G. Serum vitamin D level and bone mineral density in premenopausal Egyptian women with fibromyalgia. Rheumatol Int. 2013 (Jan);33(1):185-92. doi: 10.1007/s00296-012-2361-0.
28. Heidari B, Shirvani JS, Firouzjahi A, et al. Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis. 2010 (Oct);13(4):340-6. doi: 10.1111/j.1756-185X.2010.01561.x.
29. Çidem M, Karacan İ, Beytemur O, Kara S. Prevalence and risk factors for vitamin D deficiency in patients with widespread musculoskeletal pain. Turk J Med Sci. 2017 (Jun)12;47(3):728-731. doi: 10.3906/sag-1508-30.
30. Zhou P, Hu J, Xi P, et al. Survey on the levels of 25-hydroxy vitamin D and bone metabolic markers and evaluation of their correlations with osteoporosis in perimenopausal woman in Xi’an region. PLoS One. 2017 (Jul)7;12(7):e0180366. doi: 10.1371/journal.pone.0180366.eCollection 2017. Received 12.09.2017
/11_2.jpg)
/12.jpg)
/12_3.jpg)

/11.jpg)
/12_2.jpg)
/13.jpg)
/15.jpg)
/14_2.jpg)
/16.jpg)