Introduction
Excessive amount of thyroid hormones in the blood leads to disturbances in various organs and systems as well as to the clinical manifestations of thyrotoxicosis. The main clinical symptoms of thyrotoxicosis are due to increased secretion of thyroxine (T4) and triiodothyronine (T3), enhanced sensitivity to catecholamines. Excessive synthesis of T4 and T3 causes an increase in body metabolism, caloric effect, and fat and protein catabolism, which leads to numerous clinical signs, as it predominantly affects the cardiovascular system (heart rhythm disorders, tachycardia, atrial fibrillation) [1, 2]. The heart rate increases to 90–150 bpm (even at rest and asleep). Thyrotoxicosis is accompanied by resistance arterioles dilation and blood circulation decentralization. It has been proved by I. Klein and S. Danzi [3] that hyperthyroidism negatively affects cardiovascular haemodynamics and results in heart failure with high cardiac output, and, at late sta–ges, in dilated cardiomyopathy. The work of P.M. Osu–na et al. [4] shows that early and effective hyperthyroi–dism treatment can prevent congestive heart failure; the atrial fibrillation management and prevention of thromboembolic events are essential aspects of hyperthyroidism treatment. According to meta-analysis [5] total thyroidectomy has advantages over the antithyroid therapy for the long-term thyrotoxicosis management.
The multiplicity of target organ damages among the patients with thyrotoxicosis, indecisive benefits of inhalation anaesthesia (IA) or total intravenous anaesthesia (TIVA) in thyroidectomy, advantages or disadvantages of regional anaesthesia, such as bilateral superficial cervical plexus block (BSCPB) [6–8], the lack of data about the IA and TIVA effects on central haemodynamics in thyrotoxicosis patients during thyroid surgery require further research, improvement of anaesthetic management in the perioperative period, and the development of a patient-oriented approach [9].
The purpose of the study: to improve the results of –anaesthesia organisation in surgical treatment of thyrotoxicosis patients by choosing the anaesthesia method and optimizing perioperative anaesthesia. To achieve this aim, the following tasks were accomplished: analysis of the anaesthetic risks, the incidence of concomitant cardiac and non-cardiac pathology, the anaesthesia organisation peculiarities and the course of anaesthesia in patients with thyrotoxicosis who underwent thyroi–dectomy in a specialised endocrinology centre by analy–sing the patient’s history and anaesthetic maps; analysis of the parameters of central haemodynamics in patients with thyrotoxicosis during thyroidectomy with the use of IA and TIVA; comparison of the combined BSCPB anaesthesia techniques with the use of minimal flow –anaesthesia (MFA) (sevoflurane) or TIVA (propofol) and monoanaes–thesia; evaluation of the pharmacoeconomic aspects of minimal flow anaesthesia/low flow anaes–thesia (LFA) with sevoflurane or TIVA with propofol.
Materials and methods
The research has been carried out at the scientific department of Ukrainian Scientific and Practical Centre of Endocrine Surgery, Transplantation of Endocrine Organs and Tissues in two stages. At the first stage, the study concentrated on retrospective (2015) and prospective analysis (2016–2017) of 440 cases and anaesthetic maps of thyrotoxicosis patients who underwent thyroid surgery in 2015–2017 in order to identify the type and incidence of concomitant pathology, the specific features of general anaesthesia depending on the type of anaesthesia (TIVA or IA) and its complications. The second stage included a prospective study for the period from January 2016 to September 2017 involving 175 thyrotoxicosis patients who underwent thyroidectomy. 175 patients were randomized into 2 groups (adaptive randomization). 88 patients formed a group of balanced analgesia (BA), where a balanced (multimodal) analgesia (BMMA) was used with the mandatory implementation of the BSCPB as a component of BA [10]. The control group consisted of 87 patients with thyrotoxicosis who underwent thyroidectomy with the traditional me–thod of general anaesthesia. Depending on the type of anaesthesia, the group of balanced analgesia and control group were finally divided into the following subgroups: balanced analgesia-sevoflurane (BA-S) — 44 patients, balanced analgesia-propofol (BA-P) — 44, control-sevoflurane (C-S) — 46, control-propofol (C-P) — 41 individuals. Surgical interventions were performed under general anaesthesia with mechanical ventilation in the form of LFA or MFA with sevoflurane or using traditional method — TIVA with propofol. The anaesthetic depth control was performed by bispectral index (BIS) monitoring in the BA-S and BA-P subgroups, in the control subgroups — over the clinical course and the anaesthetic inhalation concentration at the end of the exhalation (C-S subgroup).
The perioperative stage was divided into the following parts: 1 — primary examination by an anaesthesiologist; 2 — the patient arrival at the operating room (the patient is on the operating table connected to the monitor); 3 — immediately after anaesthesia induction and tracheal intubation; 4 — surgery start; 5 — thyroid removal; 6 — after wound suturing (end of surgery); 7 — 24 hours after surgery.
At all stages the physical assessment was done accor–ding to the requirements of American Society of Anaesthesiologists (ASA), the same as the haemodynamic parameters, namely: systolic (BPsys), diastolic (BPdia) and mean (MBP) blood pressure, pulse pressure (PP), heart rate, blood oxygen saturation, stroke volume and cardiac output (CO), cardiac index (CI), oxygen delivery (DO2), etc. [11, 12]. The pain level was evaluated by the visual analogue scale (VAS) 3, 6, 12, 24 hours after surgery [13]. The analgesic consumption, the incidence of postoperative nausea and vomiting (PONV) syndrome were also evaluated [14]. The time from the end of the surgery to extubation and eye opening was evaluated. The pharmacoeconomic analysis was applied to compare the cost of general anaesthesia with sevoflurane in different fresh gas flows (FGF) and propofol dosed with BIS monitoring or the stepdown regime technique [15–18].
Results
The evidence from this study suggest that the overwhelming majority of thyrotoxicosis patients under–going thyroidectomy (67.7 %) are persons with diffuse toxic goitre (DTG), 23.6 % — with multinodular goitre (MNG) and thyrotoxicosis syndrome; 5.0 % — with toxic thyroid adenoma; the rest were the patients with recurrent DGT or MNG and individuals with combined thyroid pathology (thyroid cancer due to DGT or toxic MNG). According to ASA only 105 patients (23.9 %) of the total amount had a minimal anaesthetic risk — ASA I, the rest — ASA II–IV. The number of patients with ASA III was as follows: 33.7 % in the MNG group, 50.0 % in the MNG group with papillary cancer and 13.8 % in the group with DTG. Among 440 procedures of general anaes–thesia, 349 (79.3 %) were performed in the form of low or minimal flow anaesthesia with sevoflurane, 91 (20.7 %) — TIVA with propofol. It was analyzed that anes–thesiologists performed systemic haemodynamic and cardiac rhythm correction by administering antihypertensive, antiarrhythmic, sympathomimetic drugs in 202 (45.9 %) cases, of which 160 (45.8 %) — IA with sevoflurane, 42 (46.2 %) — TIVA with propofol (p = 0.551, no statistically significant difference). Bradycardia occurred in 9.5 and 2.2 % of cases of IA and TIVA, respectively (p < 0.05). Additionally, it should be noted that arterial hypertension was detected in 18 (78.3 %) of 23 cases with IA, 9 (75.0 %) of 12 cases with TIVA (p > 0.05). Perioperative hypertension, which required medication, arose in 26.1 % and 25.3 % of cases with IA and TIVA, respectively (p > 0.05). 76.1 % of 440 patients with thyrotoxicosis had ASA II–IV class. 21.1 % of patients were at high risk — ASA III and IV. The most common concomitant disease in DTG was dismetabolic cardiomyopathy — 55.0 %, and patients with MNG and thyrotoxicosis had symptomatic hypertension or arterial hypertension — 64.3 %. The most frequent indicators were perioperative hypertension — 26.1 and 25.3 %, arterial hypertension — 6.6 and 13.2 % during IA and TIVA, respectively, bradycardia during IA with sevoflurane — 9.5 %, and hypersalivation during TIVA — 61.5 %.
The results indicate that both IA with sevoflurane and TIVA with propofol have an effect on hemodynamic parameters. The highest depression of haemodynamic parameters (BPsys, BPdia, MBP, PP, CO, CI, general peripheral vascular resistance (GPVR)) was noted at the stage 3 — after anaesthesia induction, tracheal intubation and the initiation of anaesthesia with sevoflurane or propofol. The evidence was found that IA and TIVA negatively affect DO2. Statistical discrepancies were obvious at stages 3–6. Thus, at stage 3, the highest values of DO2 were noted in the BA-P subgroup; at stages 4–6 — in the BA-S subgroup, which had statistical significance with other subgroups.
A significant difference (p < 0.01) was also observed between the BA-S and C-S subgroups, which is due to more aggressive induction of sevoflurane in the C-S subgroup. The study ensures that both LFA and MFA do not result in tissue hypoxia with the indicated FGF. There is a definite correlation between CO, CI and GPVR obtained while using Doppler echocardiography and estimated continuous cardiac output. It was found that in the subgroups of IA with sevoflurane, there was a statistically significant (p < 0.01) decrease in CO compared to TIVA at the stages of induction and beginning of the surgery. The use of the initial FGF = 2000 ml/min and MFA with FGF = 400 ml/min with basic IA in subgroup BA-S reduces DO2 to a lesser extent compared to FGF = 4000 ml/min and LFA with FGF = 500–700 ml/min in the C-S subgroup. Dosed TIVA by BIS suppresses CO to 3–4 degrees and reduces DO2 to a lesser extent (Tables 1–4).
/557-1.jpg)
The intraoperative consumption of analgesics, in particular fentanyl, is significantly (p < 0.05) statistically lo–wer when MFA/LFA with sevoflurane is administered than in combined anaesthesia using BSCPB. The comparison of monoanaesthesia and TIVA with propofol showed the same results. BMMA with BSCPB in addition to anaesthesia with sevoflurane or propofol reduces the need for intraoperative opioid use, in particular fentanyl, compared to controls. The combination of dexketoprofen with cyclooxygenase-3 inhibitors (paracetamol or metamizole) in the BA-S and BA-P subgroups maintained long-term anaesthetic and analgesic effects of BSCPB and allowed the use of a significantly (p < 0.05) lower dose of dexketoprofen: according to the Wilcoxon criterion the use of dexketoprofen was significantly lower (p < 0.05) during the first 24 postoperative hours in the BA-S (124.6 ± 4.8 mg) and BA-P (122.4 ± 4.6 mg) subgroups compared to controls, where it was 146.8 ± 6.5 and 142.5 ± 5.8 mg in the C-S and C-P subgroups, respectively.
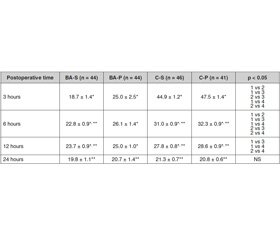
The BMMA using BSCPB, dexamethasone and dexketoprofen was more effective than traditional multicomponent anaesthesia with narcotic and non-narcotic analgesics in control subgroups, regardless the basic anaesthesia type (inhalation or TIVA). The BA-S subgroup had the best postoperative anaesthesia level, a significantly (p < 0.05) lower pain score (VAS) in the first 6 hours compared with other subgroups; significant (p < 0.05) difference in Wilcoxon criterion regarding the pain level by VAS remained in the BA and control subgroups for 12 hours after surgery (the level of VAS was lower in the BA subgroups) (Table 5).
Discussion
Thus, the BA-S subgroup had the best level of post–operative anaesthesia with significantly (p < 0.05) lower pain (VAS) in the first 6 hours compared to other subgroups; significant (p < 0.05) difference in VAS score (Wilcoxon test) maintained between the BA and C-S and C-P subgroups for 12 hours after surgery (VAS score was lower in both BA subgroups). Better nociceptive monitoring with combined anaesthesia contributes to significantly lower intraoperative blood loss — 44.03 ± 3.71 ml compared to 69.57 ± 4.47 ml in monoanaesthesia. The best antiemetic scheme was proved to be the use of BA combined with basic anaesthesia with propofol in the BA-P subgroup with the addition of metoclopramide before anaesthesia induction. The highest number of patients who did not experience PONV was 77.3 %. BSCPB use leads to lower incidence of PONV. The lowest PONV values were no–ted in the BA-P subgroup — the overall rate of PONV was 22.7 %, clinically significant PONV — only 9.1 %. The difference in the PONV frequency in the BA-P subgroup was statistically insignificant, but was clinically lower (by 4.6 %) than the overall PONV frequency in the BA-S subgroup (27.3 %). The lowest incidence of clinically significant PONV was 9.1 %, the lowest overall PONV score was 0.36 ± 0.11 (all indicators were significantly lower than in the control subgroups, C-S and C-P). The findings confirmed that both the time before extubation and the time before eye opening in the subgroups were statistically significant (p < 0.001) in TIVA compared to IA, both with BIS monitoring and without it. Indicators of both extubation and eye ope–ning time in subgroups were significantly (p < 0.001) statistically lower in TIVA compared to IA, with BIS monitoring and without it. It has been investigated that in BIS monitoring compared with the end-tidal anaesthetic concentration monitoring, the average time from wound suturing to extubation and time to eye ope–ning after extubation were significantly lower (p < 0.01) in the BA-S subgroup (9.21 ± 0.73 min and 3.21 ± 0.12 min, respectively) compared to C-S (12.63 ± 0.62 min and 6.34 ± 0.15 min). In TIVA subgroups, while using BIS monitoring, the mean time from wound suturing to extubation was 8.63 ± 0.62 min and 11.41 ± 0.82 min in the BA-P and C-P subgroups, respectively (the difference was significant, p = 0.0083). The time of eye opening after extubation was 2.81 ± 0.14 min in the BA-P subgroup and 4.56 ± 0.13 min in the C-P subgroup (the difference was significant, p < 0.0001).
Individual approach to the inhaled and intravenous anaesthetic administration and correction under the control of BIS monitoring give an opportunity to personalize general anaesthesia.
The study also demonstrated a statistically significant (p < 0.05) decrease in the intraoperative analgesic consumption in MFA/LFA with sevoflurane (BA-S — 283.4 ± 12.4 μg/per operation and C-S — 376.9 ± 12.9 μg) compared to TIVA with propofol (BA-P — 353.4 ± 15.4 μg and C-P — 443.7 ± 19.0 μg, respectively). The values of the lo–west (BA-S subgroup) and the highest (C-P subgroup) fentanyl consumption were significantly different in 3 other subgroups. The anaesthetic costs were lowest in the BA-S subgroup, where the minimum FGF was 400 ml/min, and the initial FGF = 2000 ml/min, the cost of anaesthesia was 170.2 ± 4.2 UAH (Dion’s equation), 183.3 ± 4.1 UAH (EMM calculation). The low-flow IA in the C-S subgroup (FGF = 500 ml/min) costed 186.2 ± 6.6 UAH (Dion’s equation) and 203.8 ± 7.8 UAH (EMM calculation), and have significant economic advantages (p < 0.05) compared to TIVA with propofol. When using FGF = 700 ml/min, the expenses for IA (Dion’s equation — 226.8 ± 8.6 UAH, EMM — 258.2 ± 7.8 UAH) and TIVA with propofol (231.4 ± 5.1 UAH and 238.8 ± 5.2 UAH, respectively, in the BA-P and C-P subgroups, p = 0.3808) did not show a statistically significant difference. In the C-S subgroup with the use of FGF = 700 ml/min (n = 21), the costs were 226.8 ± 8.6 UAH (Dion’s equation), and 258.2 ± 7.8 UAH (EMM).
Conclusions
The study provides considerable evidence for the modern thyroid surgery. The findings confirm the improvement of surgical treatment in thyrotoxicosis patients by choosing the method of anaesthesia and optimizing the perioperative anaesthesia. The new reliable data obtained allowed solving the current problem of anaesthesia management in thyrotoxicosis patients. The evidence from this study points towards the idea of a personified approach to the general anaesthesia and perioperative –anaesthesia choice using a combination of balanced analgesia and modern methods for anaesthesia control.
The results of this study indicate that thyrotoxicosis deteriorates the physical status of the patient according to ASA. 76.1 % of 440 patients with thyrotoxicosis had ASA II–IV class. 21.1 % of patients were at high risk — ASA III and IV.
The study highlights the negative impact of IA with sevoflurane and TIVA with propofol on the hemodynamics and oxygen delivery.
Our investigations in this field seem likely to confirm the hypothesis that combined anaesthesia with BSCPB performed as a pre-emptive analgesia using MFA with sevoflurane or TIVA with propofol provides statistically (p < 0.05) significant improvement in pain relief compared to monoanaesthesia.
The study also demonstrates that the intraoperative analgesic consumption is significantly lower in MFA/LFA with sevoflurane compared to TIVA with propofol.
Conflicts of interests. Authors declare no conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Паньків В.І. Практична тиреоїдологія. — Донецьк: Видавничий дім «Заславський», 2011. — 224 с.
2. Черенько М.С. Сучасні погляди на діагностку та лікування гіпертиреозу та інших форм тиреотоксикозу: огляд останніх рекомендацій Американської тиреоїдної асоціації (2016) // Клінічна ендокринологія та ендокринна хірургія. — 2016. — № 4(56). — С. 87-93. http://doi.org/10.24026/1818-1384.4(56). 2016.87324.
3. Klein I., Danzi S. Thyroid disease and the heart // Current Problem in Cardiology. — 2016. — Vol. 41(2). — P. 65-92. doi: 10.1016/j.cpcardiol.2015.04.002.
4. Osuna P.M., Udovcic M., Sharma M.D. Hyperthyroidism and the Heart // Methodist DeBakey Cardiovascular Journal. — 2017. — Vol. 13(2). — P. 60-63. http://doi.org/10.14797/mdcj-13-2-60.
5. Sundaresh V., Brito J.P., Wang Z., Prokop L.J., Stan M.N., Murad M.H., Bahn R. Comparative Effectiveness of Therapies for Graves’ Hyperthyroidism: A Systematic Review and Network Meta-Analysis // The Journal of Clinical Endocrinology & Metabolism. — 2013. — Vol. 98(9). — P. 3671-3677. https://doi.org/10.1210/jc.2013-1954.
6. Kale S., Shipra A., Vineet S. Evaluation of the Analgesic Effect of Bilateral Superficial Cervical Plexus Block for Thyroid Surgery: A Comparison of Presurgical with Postsurgical Block // Indian Journal of Surgery. — 2015. — Vol. 77(3). — P. 1196-1200.
7. Hall G.M., Hunter J.M., Cooper M.S. Core Topics in Endocrinology in Anaesthesia and Critical Care. — Cambridge University Press: Cambridge, United Kingdom, 2010. — 202 p. ISBN: 978-0-521-50999-2.
8. Bajwa S.J.S., Sehgal V. Anesthesia and thyroid surgery: The never ending challenges // Indian Journal of Endocrinology and Metabolism. — 2013. — Vol. 17(2). — P. 228-234. http://doi.org/10.4103/2230-8210.109671.
9. Purdon P.L., Zhou D.W., Akeju O., Brown E.N. The electroencephalogram: a missing piece in personalized anesthesia care // Anesthesiology. — 2015. — Vol. 123(3). — P. 725-728. http://doi.org/10.1097/ ALN.0000000000000794.
10. Ларін О.С. Анестезіологічний менеджмент тиреоїдектомій у пацієнтів з тиреотоксикозом: оптимізация опіоїд-зберегаючого та антиеметичного компонентів [Текст] / О.С. Ларін, С.М. Черенько, С.О. Тарасенко, С.О. Дубров, М.Б. Горобейко, І.О. Куліш // Біль, знеболення та інтенсивна терапія. — 2016. — № 2. — С. 5-18. doi: http://dx.doi.org/10.25284/2519-2078.2(75).2016.83981.
11. Permpikul C., Leelayuthachai T. Non-invasive estimated continuous cardiac output (escCO) during severe sepsis and septic shock resuscitation // Journal of Medical Association of Thailand. — 2014. — Vol. 97(3). — P. 184-188.
12. Terada T., Oiwa A., Maemura Y. et al. Comparison of the ability of two continuous cardiac output monitors to measure trends in cardiac output: estimated continuous cardiac output measured by modified pulse wave transit time and an arterial pulse contour-based cardiac output device // Journal of Clinical Monitoring and Computing. — 2015. — Vol. 30(5). — P. 621-627. doi: 10.1007/s10877-015-9772-x.
13. Boswell M.V., Cole B.E. Weiner’s pain management: a practical guide for clinicians. — 7th ed. — CRC Press, Taylor & Francis Group: Boca Raton, FL, 2006. — 1612 p.
14. Apfel C.C., Laara E., Koivuranta M., Greim C.A., Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers // Anesthesiology. — 1999. — Vol. 91(3). — P. 693-700. doi: 10.1097/00000542- 199909000-00022.
15. Лісний І.І., Закальська Х.А., Стрепетова О.В. Економічні складові різних видів анестезії // Хірургія України. — 2016. — № 1. — С. 103-108.
16. Biro P. Calculation of volatile anaesthetics consumption from agent concentration and fresh gas flow // Acta anaesthesiologica Scandinavica. — 2014. — Vol. 58(8). — P. 968-972. doi: 10.1111/aas.12374.
17. Dion P. The cost of anaesthetic vapours // Canadian Journal of Anaesthesia. — 1992. — Vol. 39(6). — P. 633-633.
18. Тарасенко С.О. Витрати інгаляційного анестетика: методи оцінки та кореляція між ними / С.О. Тарасенко, С.О. Дубров, М.В. Кунатовський, В.А. Смоляр // –Біль, знеболення та інтенсивна терапія. — 2017. — № 1. — С. 5-18. doi: http://dx.doi.org/10.25284/2519-2078.1(78). 2017.103518.

/557-1.jpg)
/558-1.jpg)