Международный эндокринологический журнал Том 15, №2, 2019
Вернуться к номеру
Оцінка соматотропної функції в дітей із синдромом біологічно неактивного гормонa росту на основі проведення стимуляційних тестів iз клонідином та інсуліном
Авторы: Sprinchuk N.A.
State Institution “V.P. Komisarenko Institute of Endocrinology and Metabolism of NAMS of Ukraine”, Kyiv, Ukraine
Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine
Рубрики: Эндокринология
Разделы: Справочник специалиста
Версия для печати
. Мета дослідження: оцінити ефективність фармакологічних тестів iз клонідином та інсуліном для стимуляції соматотропного гормона (СТГ) при діагностиці синдрому біологічно неактивного гормонa росту (СБНГР). Матеріали та методи. Обстежено 158 хворих на СБНГР — 47 дівчаток і 111 хлопчиків, середній вік яких становив 8,30 ± 0,24 року. У дослідження включені пацієнти з відставанням у рості понад 2 стандартнi вiдхилення. Визначали базальний та стимульований рівні СТГ із застосуванням тестів з інсуліном та клонідином. Обов’язково проводили чотириденну пробу на чутливість до гормонa росту (ГР) з визначенням рівнів інсуліноподібного фактора росту1 до першої ін’єкції ГР і наступного дня після завершення проби. Результати. Максимальне підвищення рiвня СТГ на тлі тестів з інсуліном та клонідином в усіх пацієнтів iз СБНГР становило понад 10 нг/мл. Максимальне збільшення показників СТГ було вірогідно вищим (р < 0,01) при проведенні стимуляційного тесту з клонідином, ніж з інсуліном. Це доведено як у дітей з СБНГР — 17,79 ± 0,51 нг/мл та 13,83 ± 0,92 нг/мл відповідно, так і в контрольній групі — 16,81 ± 1,60 нг/мл і 11,18 ± 0,70 нг/мл. Зафіксовано, що індекс стандартного відхилення інсуліноподібного фактора росту1 у хворих дітей вірогідно нижчий (р < 0,001), ніж у контрольнiй групi. Більш вираженими зміни були в пацієнтів препубертатного віку. Висновки. Фармакологічний тест iз клонідином є більш інформативним, ніж з інсуліном, і викликає вірогiдно вищий стимуляційний максимальний викид ГР. Рекомендовано розпочинати дослідження функції СТГ з проведення проби з клонідином, що є більш безпечною завдяки відсутності ризику тяжких гіпоглікемічних станів.
Цель исследования: оценить эффективность фармакологических тестов с клонидином и инсулином для стимуляции соматотропного гормона (СТГ) при диагностике синдрома биологически неактивного гормона роста (СБНГР). Материалы и методы. Обследовано 158 больных с СБНГР — 47 девочек и 111 мальчиков, средний возраст которых составил 8,30 ± 0,24 года. В исследование включены пациенты с отставанием в росте более 2 стандартных отклонений. Определяли базальный и стимулированный уровни СТГ с применением тестов с инсулином и клонидином. Обязательно проводили четырехдневную пробу на чувствительность к гормону роста (ГР) с определением уровней инсулиноподобного фактора роста1 до первой инъекции ГР и на следующий день после завершения пробы. Результаты. Максимальное повышение уровня СТГ на фоне тестов с инсулином и клонидином у всех пациентов с СБНГР составляло более 10 нг/мл. Максимальное увеличение показателей СТГ было достоверно более высоким (р < 0,01) при проведении стимуляционного теста с клонидином, чем с инсулином. Это доказано как у детей с СБНГР — 17,79 ± 0,51 нг/мл и 13,83 ± 0,92 нг/мл соответственно, так и в контрольной группе — 16,81 ± 1,60 нг/мл и 11,18 ± 0,70 нг/мл. Зафиксировано, что индекс стандартного отклонения инсулиноподобного фактора роста1 у больных детей достоверно ниже (р < 0,001), чем в контрольной группе. Более выраженные изменения отмечены у пациентов препубертатного возраста. Выводы. Фармакологический тест с клонидином является более информативным, чем с инсулином, и вызывает достоверно более высокий стимуляционный максимальный выброс ГР. Рекомендуется начинать исследования функции СТГ с проведения пробы с клонидином, которая является более безопасной благодаря отсутствию риска тяжелых гипогликемических состояний.
Background. Growth pathology caused by somatotropic insufficiency is one of the most urgent problems in pediatric endocrinology. An increase in the growth hormone (GH) level less than 10 ng/ml was traditionally considered a criterion for diagnosing somatotropic insufficiency in patients with short stature, when performing provocation tests. The purpose is to evaluate the effectiveness of clonidine and insulin provocation tests for the somatotropic hormone (STH) stimulation in diagnosing the syndrome of biologically inactive growth hormone (SBIGH). Materials and methods. A total of 158 patients with SBIGH (47 girls and 111 boys aged 8.30 ± 0.24 years) were examined. The study included patients with delayed growth more than 2 standard deviations. Basal and stimulated STH levels were determined using insulin and clonidine tests. A 4-day growth hormone sensitivity test was obligatorily performed for determining insulin-like growth factor 1 levels prior to the first GH injection and the day after the test completion. Results. The maximum increase in STH against a background of insulin and clonidine tests was above 10 ng/ml in all patients with SBIGH. Significantly higher maximum increase of STH parameters (p < 0.01) was noted in clonidine stimulation test than in insulin one. This was proved both in patients with SBIGH (17.79 ± 0.51 ng/ml and 13.83 ± 0.92 ng/ml, respectively) and in children of control group (16.81 ± 1.60 and 11.18 ± 0.70 ng/ml). Standard deviation score of insulin-like growth factor 1 was significantly lower (p < 0.001) in children with SBIGH than in control group. More pronounced changes were observed in prepubertal patients. Conclusions. Clonidine provocation test is more informative than insulin one, and causes a significantly higher stimulating maximum release of GH. It is recommended to start the investigation of STH function with clonidine test, which is safer for patients due to the absence of a risk of severe hypoglycemic conditions.
синдром біологічно неактивного гормона росту; тест з інсуліном; тест iз клонідином; соматотропний гормон
синдром биологически неактивного гормона роста; тест с инсулином; тест с клонидином; соматотропный гормон
syndrome of biologically inactive growth hormone; insulin test; clonidine test; somatotropic hormone
Introduction
Growth pathology caused by somatotropic insufficiency is one of the most urgent problems in pediatric endocrinology. An increase in the growth hormone (GH) level less than 10 ng/ml was traditionally consi–dered a criterion for diagnosing somatotropic insufficiency in patients with short stature, when performing provocation tests [1, 2].
The study of the insulin–like growth factor 1 (IGF–1) level is mandatory for the complete diagnosis, but not the main one in patients with the somatotropic hormone (STH) deficiency. Factors that affect the decrease of its level, except GH, may be diverse, such as enzymopathy, hepatitis and hepatosis, celiac disease, other somatic and psychological problems, etc. [3, 4]. Therefore, the main criteria for diagnosing the GH deficiency is short sta–ture, along with a low level of STH release against a background of provocation tests. There is a group of diseases, including the syndrome of biologically inactive growth hormone (SBIGH), that are accompanied by short sta–ture against a background of normal indicators of STH release, when stimulation tests are performed.
The basic diagnostic laboratory criteria for SBIGH are low IGF–1 levels under normal, even elevated level of STH and positive GH test [5, 6]. Data on the specifi–city and sensitivity of STH receptor to stimulating action of the clonidine and insulin provocation agents, which are the gold standard for the GH stimulation [7, 8], have not been found in patients with SBIGH in domestic and foreign literature, which served as the basis for this study.
The purpose of the study is to evaluate the effectiveness of clonidine and insulin provocation tests for somatotropic hormone stimulation in diagnosing the syndrome of biologically inactive growth hormone.
Materials and methods
A total of 158 patients with SBIGH (47 girls and 111 boys aged 8.30 ± 0.24 years) were examined at the Department of Pediatric Endocrine Pathology of State Institution “V.P. Komisarenko Institute of Endocrinology and Metabolism of the National Academy of Medical Sciences of Ukraine” in 2008–2018. The study included children with delayed growth of more than 2 standard deviations from the mean median of physiological age and sex parameters. Bone age was more than 2 years delayed from the age in years.
A physical examination, measurements of growth using the Harpenden stadiometer, body mass measurements by the Seca scales, and the assessment of body proportions were included in the plan for compulsory examination of the patient. Body mass index (BMI) was calculated by the formula: BMI = m/p2, where m — body weight (kg), p — height (m). The results were evaluated according to the percentile nomograms for this gender and the mean chronological age [9]. The puberty stage was assessed using Tanner scale (1962).
Hormone testing was performed in all patients, it included evaluation of basal and stimulated levels of STH, IGF–1, thyroid–stimulating hormone, free triiodthyronine, free thyroxine in the blood by radioimmunological method using standard IRMA kits (Immunotech, the Czech Republic). Insulin and clonidine tests were used to determine the stimulation level of STH by the standard methods in accordance with approved protocols of the Ministry of Health of Ukraine, which are consistent with the International consensus on the definition of somatotropic insufficiency and stimulation tests [1, 2, 10].
A 4–day GH sensitivity test was obligatorily performed in all the patients. This test consists in the introduction of a genetically engineered GH at a dose of 0.033 mg/kg/day subcutaneously for 4 days, and the determination of IGF–1 levels prior to the first injection of GH and the day after the completion of the test. The test is considered positive if the level of IGF–1 increases by 2 times or more [6].
The control group consisted of 42 healthy children of corresponding age. The statistical analysis was performed by the Microsoft Excel program using the Student’s t–test with determination of the p–value difference. The diffe–rence was considered to be significant at p < 0.05. Laboratory studies were conducted in the accredited laboratories of the institute.
Results
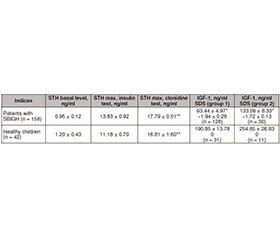
The determination of STH level in children with SBIGH revealed that its basal content was 0.95 ± 0.12 ng/ml, which varied in comparison with healthy children of the control group (average level 1.20 ± 0.43 ng/ml), but no significant difference was observed between the given indices (Table 1).
The maximum increase in STH against a background of insulin and clonidine tests was above 10 ng/ml in all patients with SBIGH. In contrast to the basal level of GH, under conditions of stimulation, levels of STH were higher in patients with SBIGH than in the control group, but no significant difference was between them. It is important to note that the maximum STH increase is significantly higher (p < 0.01) when performing a stimulation test with clonidine than with insulin. Moreover, this was proved both in children with SBIGH — 17.79 ± 0.51 ng/ml and 13.83 ± 0.92 ng/ml, respectively, and in the control group — 16.81 ± 1.60 ng/ml and 11.18 ± 0.70 ng/ml.
IGF–1 determination in children with SBIGH is extremely necessary and a mandatory criterion for diagnosis of this syndrome. However, in order to exclude hyperdiagnosis in children with malnutrition, BMI, which met age standards, was measured in all the patients [9]. A 4–day GH sensitivity test was performed in all the patients, it was included in the differential diagnostic algorithm of the SBIGH with its various forms (sensitive and insensitive to GH), with receptor insensitivity to GH (Laron syndrome), and is necessary to clarify the diagnosis of SBIGH [4]. The 4–day GH sensitivity test was positive in all the children.
IGF–1 indices have a direct correlation with age and puberty, which is the second most important factor for GH–stimulated IGF–1 synthesis [7, 8]. In this regard, when determining IGF–1 content, all children, both patients with the SBIGH and healthy individuals, were divided into two groups (groups 1 and 2). The main criterion for including children into one or another group was the stage of their puberty by the Tanner scale.
Signs of puberty were absent in 128 (81 %) of 158 patients. These prepubertal children with stage I puberty were included in group 1. Thirty children of puberty age (stages II and III by the Tanner scale) were included in group 2. Among them, 21 patients had stage II puberty, and 9 children — stage III puberty. There were no children with IV and V stages of puberty. In groups 1 and 2, IGF–1 indices have a high degree of significance (p < 0.001) below the indices of control group, which coincided with the reference values for the corresponding age groups.
If a comparative analysis is performed between sigma deviations of IGF–1 in patients with SBIGH and healthy children, it is important to note that SDS of IGF–1 in patients with SBIGH was significantly lower (p < 0.001). More pronounced changes were in prepubertal children. There were no significant differences in SDS of IGF–1 between children in groups 1 and 2.
Thus, this study proved that all patients met the laboratory criteria characteristic for the SBIGH, namely that they had normal basal and stimulated STH levels with significantly lower IGF–1 and SDS of IGF–1 and normal BMI.
In the control group, all the studied parameters, namely the levels of STH (basal and stimulated), IGF–1, and SDS of IGF–1, were within the reference values for the respective age groups.
Discussion
Today, in order to standardize the examination of children with short stature, a number of provocation tests have been developed for evaluating the level of GH–stimulated secretion. More than 30 stimulation tests have been described, among which insulin, clonidine (clophelin), arginine, glucagon, and L–dopa are the most widely used. Any of the above–mentioned stimulators of GH contributes to a significant release (over 10 ng/ml) in almost 90 % of healthy children [11–13].
Arginine and L–dopa tests in the practice of our department have not been used recently due to negative neurological manifestations, such as headache, nausea, temporary psychopathic manifestations.
According to the recent literature data, the sensitivity of the test with clonidine is 98 %, with insulin — 85–100 %. Introduction of stimulation tests for the detection of STH deficiency enables the timely diagnosis of GH insufficiency [14, 15]. According to other authors who conducted provocative tests in 120 children, 83 (69.2 %) with GH deficiency and 37 (30.8 %) with idiopathic short stature, the specificity and accuracy of the insulin test were 78.4 and 93.6 %, respectively. Specificity and accuracy of L–dopa stimulation test were 29.7 and 79.2 %, respectively [16].
Diagnostic value of a provocative test with insulin (0.075 unit/kg intravenously) in combination with clonidine (4 µg/kg orally) revealed its significantly higher specificity (74, 88%) compared to insulin (48 %) or clonidine (65 %). Accuracy of insulin + clonidine test was also better (75, 85 %) compared to insulin (63 %) and clonidine (73 %) tests. The authors concluded that a combined test with clonidine and insulin is expedient, convenient, time–saving and reliable tool compared to clonidine or insulin tests alone [13]. However, the simultaneous use of two tests in one patient has contraindications, especially in children under the age of 5 years.
That is, the literature data showed that clonidine test is more informative than insulin one, but these findings are obtained in patients with GH insufficiency, idiopa–thic short stature and healthy children. So, we conducted a study in children with SBIGH and significantly confirmed that maximum increase in GH was higher when using stimulation test with clonidine than with insulin.
Conclusions
1. Stimulation test with clonidine is more informative than with insulin and causes a significantly higher maximum STH release, both in patients with SBIGH and in healthy children.
2. It is recommended to start a study of the STH function with clonidine test that is safer for patients due to the absence of a risk of severe hypoglycaemic conditions, which particularly occur when using insulin stimulation test in young children with low BMI and predisposition to lower level of fasting glucose in patients with high risk of hypopituitarism.
Conflicts of interests. Author declares no conflicts of interests that might be construed to influence the results or interpretation of their manuscript.

/149-1.jpg)