Международный эндокринологический журнал Том 17, №1, 2021
Вернуться к номеру
Поширеність неалкогольної жирової хвороби печінки у хворих на предіабет
Авторы: Bhumi Agarwal, B.M. Singh Lamba, Neera Sharma, Monika Tanwar
ABVIMS and Dr RML Hospital, New Delhi, India
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Актуальність. Цукровий діабет (ЦД) є одним із глобальних і надзвичайних викликів у галузі охорони здоров’я. Предіабет — це рання стадія в континуумі гіперглікемії, при якій організм схильний до підвищеного ризику розвитку ЦД. Неалкогольна жирова хвороба печінки (НАЖХП) становить собою низку порушень функції печінки, які характеризуються стеатозом печінки або накопиченням жиру в клітинах печінки за відсутності надмірного вживання алкоголю, вірусної етіології чи вживання наркотиків. На сьогодні проведено не так багато досліджень із метою вивчення поширеності НАЖХП в осіб із предіабетом. Дане дослідження є зусиллям у цьому напрямку. Матеріали та методи. Проведене поперечне обсерваційне дослідження. 100 пацієнтів із предіабетом були включені в дослідження протягом періоду з листопада 2017 року по березень 2019 року після отримання інформованої згоди. Дані дослідження включали визначення біохімічних показників, рівня глікемії в плазмі натще, електролітів та HbA1c, інсуліну в сироватці крові, проведення загального аналізу крові, функціональних тестів печінки та нирок, 2-годинного орального глюкозотолерантного тесту. Результати. Дослідження включало 38 чоловіків та 62 жінки, середній вік досліджуваної популяції становив 46 років. Установлено, що середній індекс маси тіла (ІМТ) становить 24,29 ± 3,98 кг/м2, окружність талії — 81,26 ± 8,71 см. Виявлений вірогідний зв’язок між показниками ехоструктури печінки при ультразвуковому дослідженні (УЗД) та ІМТ (р = 0,003) і статтю. Установлено, що 30 % обстежених мають порушену чутливість до інсуліну, у 22 % виявлені ранні прояви резистентності до інсуліну, у 48 % — виражена інсулінорезистентність. Показано статистично значущу кореляцію між результатами УЗД й еластографії. Виявлено статистичну кореляцію між HOMA-IR та показниками ехоструктури на УЗД, а також середньою жорсткістю печінки при еластографії. Висновки. Виявлено вірогідну кореляцію між резистентністю до інсуліну та наявністю НАЖХП. Спостерігалися вірогідні зв’язки між різними демографічними характеристиками та ступенем стеатозу. Існує необхідність провести подальші дослідження в більш широкому масштабі, щоб обґрунтувати отримані результати цього дослідження. Очікується, що це значною мірою сприятиме підвищенню обізнаності й оптимізації стратегій охорони здоров’я.
Актуальность. Сахарный диабет (СД) — один из глобальных и чрезвычайных вызовов в области здравоохранения. Предиабет — это ранняя стадия в континууме гипергликемии, при которой организм подвержен повышенному риску развития СД. Неалкогольная жировая болезнь печени (НАЖБП) представляет собой ряд заболеваний печени, характеризующихся стеатозом печени или накоплением жира в клетках печени при отсутствии чрезмерного употребления алкоголя, вирусной или лекарственной этиологии. На сегодняшний день проведено недостаточно исследований для изучения распространенности НАЖБП у лиц с предиабетом. Данное исследование является попыткой в этом направлении. Материалы и методы. Проведено перекрестное обсервационное исследование. 100 пациентов с предиабетом были включены в исследование в период с ноября 2017 года по март 2019 года после получения информированного согласия. Данные исследования включали определение биохимических показателей, уровня гликемии в плазме натощак, электролитов и HbA1c, инсулина в сыворотке крови, проведение общего анализа крови, функциональных тестов печени и почек, 2-часового орального глюкозотолерантного теста. Результаты. Исследование включало 38 мужчин и 62 женщины, средний возраст исследуемой популяции составлял 46 лет. Установлено, что средний индекс массы тела составил 24,29 ± 3,98 кг/м2, а окружность талии — 81,26 ± 8,71 см. Обнаружена достоверная связь между уровнем эхоструктуры при ультразвуковом исследовании (УЗИ) и ИМТ (p = 0,003) и полом. Установлено, что 30 % обследованных имеют нарушенную чувствительность к инсулину, 22 % имели ранние ее признаки и 48 % — выраженную резистентность к инсулину. Показана статистически значимая корреляция между результатами УЗИ и эластографии. Выявлена статистическая корреляция между HOMA-IR и показателями эхоструктуры на УЗИ, а также средней жесткостью печени при эластографии. Выводы. Обнаружена достоверная корреляция между инсулинорезистентностью и наличием НАЖБП. Наблюдались достоверные ассоциации между различными демографическими характеристиками и степенью стеатоза. Необходимо провести дальнейшие исследования в более крупном масштабе, чтобы подтвердить наблюдения этого исследования. Ожидается, что это в значительной степени будет способствовать повышению осведомленности и оптимизации стратегий общественного здравоохранения.
Background. Diabetes mellitus (DM) is one of the largest global health emergencies. Prediabetes is an early stage in hyperglycemia continuum where individual is at an increased risk for development of DM. NAFLD represents a range of liver disorders characterized by hepatic steatosis or accumulation of fat in the liver cells in the absence of excessive alcohol consumption, viral or drug related etiologies. However, not many studies have been conducted to study the prevalence of non-alcoholic fatty liver disease (NAFLD) in persons with prediabetes. This study is an endeavor in that direction. Materials and methods. This was a cross-sectional observational study. 100 prediabetic patients, fulfilling the criteria as under, were included in the study over a period from November 2017 to March 2019, after informed consent. Investigations carried out on the patients included baseline biochemical parameters like complete hemogram, fasting plasma glucose, liver function tests, kidney function tests, serum electrolytes and specialized investigations like HbA1c, 2-hour-OGTT and serum insulin levels. Results. The study included 38 males and 62 females, with the median age for the study population being 46 years. The mean BMI was found to be 24.29 ± 3.98 kg/m2, and the mean waist circumference was found to be 81.26 ± 8.71 cm. A significant association was found between the level of fatty echotexture on ultrasound and BMI (p = 0.003), and gender (0.05). 30 % population was found to be insulin sensitive, 22 % was found to be depicting early insulin resistance and 48 % had significant insulin resistance. There was a statistically significant correlation between ultrasound and fibroscan findings. A significant statistical correlation was found between HOMA IR and level of fatty echotexture on ultrasound, as well as median liver stiffness on fibroscan. Conclusions. We found a significant correlation between insulin resistance and presence of NAFLD. Also, significant associations were observed between various demographic characteristics and grade of steatosis. There is a need to undertake further studies on a larger scale, to substantiate the observations of this study. This understanding is expected to go a long way in generating awareness and optimizing public health strategies.
предіабет; неалкогольна жирова хвороба печінки; поширеність
предиабет; неалкогольная жировая болезнь печени; распространенность
prediabetes; non-alcoholic fatty liver disease; prevalence
Introduction
Materials and methods
Statistical analysis
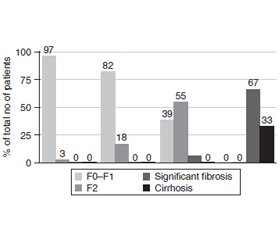
Results
/37.jpg)
Discussion
Conclusions
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S. et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res. Clin. Pract. 2019. 157. 107843. doi: 10.1016/j.diabres.2019.107843.
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes — 2021. American Diabetes Association. Diabetes Care. 2021. 44(Suppl. 1). 15-33. DOI: 10.2337/dc21-S002.
- Wu W.C., Wei J.N., Chen S.C., Fan K.C., Lin C.H., Yang C.Y., Lin M.S. et al. Progression of insulin resistance: A link between risk factors and the incidence of diabetes. Diabetes Res. Clin. Pract. 2020. 161. 108050. doi: 10.1016/j.diabres.2020.108050.
- Neuschwander-Tetri B.A. Therapeutic Landscape for NAFLD in 2020. Gastroenterology. 2020. 158(7). 1984-1998. e3. doi: 10.1053/j.gastro.2020.01.051.
- Taylor R.S., Taylor R.J., Bayliss S., Hagström H., Nasr P., Schattenberg J.M., Ishigami M. et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020. 158(6). 1611-1625. e12. doi: 10.1053/j.gastro.2020.01.043.
- Ortiz-Lopez C., Lomonaco R., Orsak B., Finch J., Chang Z., Kochunov V.G., Hardies J., Cusi K. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care. 2012. 35(4). 873-878. doi: 10.2337/dc11-1849.
- Mansour-Ghanaei F., Jafarinejad A., Roshan Z.A., Khosravi H., Hassankhani A., AmirMaafi A. Non-alcoholic fatty liver disease and prediabetes. J. Pioneer Med. Sci. 2016. 6(1). 6-9.
- Stefan N., Staiger H., Wagner R., Machann J., Schick F., Häring H.U., Fritsche A. A high-risk phenotype associates with reduced improvement in glycaemia during a lifestyle intervention in prediabetes. Diabetologia. 2015. 58(12). 2877-2884. doi: 10.1007/s00125-015-3760-z.
- Mohan V., Farooq S., Deepa M., Ravikumar R., Pitchumoni C.S. Prevalence of non-alcoholic fatty liver disease in Urban South Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Research and Clinical Practice. 2009. 84(1). 84-91. DOI: 10.1016/j.diabres.2008.11.039.
- Suresh S., Rajanbabu B., Veetil V.M., Hussain A., Veetil J.N. A study on the altered glycemic and lipid parameters and prevalence of insulin resistance in nonalcoholic fatty liver disease. J. Family Med. Prim. Care. 2018. 7(1). 93-97. doi: 10.4103/jfmpc.jfmpc_352_16.
- Bugianesi E., Gastaldelli A., Vanni E. et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005. 48. 634-642. doi: 10.1007/s00125-005-1682-x.
- Nassir F., Rector R.S., Hammoud G.M., Ibdah J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol (NY). 2015. 11(3). 167-175. PMID: 27099587; PMCID: PMC4836586.
- Xia W., Pessentheiner A.R., Hofer D.C., Amor M. et al. Loss of ABHD15 Impairs the Anti-lipolytic Action of Insulin by Altering PDE3B Stability and Contributes to Insulin Resistance. Cell. Rep. 2018. 23(7). 1948-1961. doi: 10.1016/j.celrep.2018.04.055.
- Rhee E.J., Lee W.Y., Cho Y.K., Kim B.I., Sung K.C. Hyperinsulinemia and the development of nonalcoholic Fatty liver disease in nondiabetic adults. Am. J. Med. 2011. 124(1). 69-76. doi: 10.1016/j.amjmed.2010.08.012.
- Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012. 55(6). 2005-2023. doi: 10.1002/hep.25762.
- Zhang G., Zhao Q., Lin C., Hu Z., Zhang T., Gao Z. Transient Elastography and Ultrasonography: Optimal Evaluation of Liver Fibrosis and Cirrhosis in Patients with Chronic Hepatitis B Concurrent with Nonalcoholic Fatty Liver Disease. BioMed Research International. 2019. Article ID 3951574. 10 p. https://doi.org/10.1155/2019/3951574.
- Bedogni G., Miglioli L., Masutti F., Tiribelli C., Marchesini G., Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005. 42(1). 44-52. doi: 10.1002/hep.20734.
- Summart U., Thinkhamrop B., Chamadol N., Khuntikeo N., Songthamwat M., Kim C.S. Gender differences in the prevalence of nonalcoholic fatty liver disease in the Northeast of Thailand: A population-based cross-sectional study. F1000Res. 2017. 6. 1630. Published 2017, Oct 20. DOI: 10.12688/f1000research.12417.1.
- Liu M., Wang J., Zeng J., Cao X., He Y. Association of NAFLD With Diabetes and the Impact of BMI Changes: A 5-Year Cohort Study Based on 18,507 Elderly. J. Clin. Endocrinol. Metab. 2017. 102(4). 1309-1316. doi: 10.1210/jc.2016-3440.
- Privitera G., Spadaro L., Alagona C., Calanna S., Piro S., Rabuazzo A.M., Purrello F. Hepatic insulin resistance in NAFLD: relationship with markers of atherosclerosis and metabolic syndrome components. Acta Diabetol. 2016. 53(3). 449-459. DOI: 10.1016/j.dld.2015.07.026.
- Li M., Zhang S., Wu Y., Ye J., Cao X., Liu J., Sun Y., Zhong B. Prevalence of Insulin Resistance in Subjects with Nonalcoholic Fatty Liver Disease and Its Predictors in a Chinese Population. Dig. Dis. Sci. 2015. 60(7). 2170-2176. DOI: 10.1007/s10620-015-3564-5.
- Singh Y., Garg M.K., Tandon N., Marwaha R.K. A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban Indian adolescents. J. Clin. Res. Pediatr. Endocrinol. 2013. 5(4). 245-251. doi: 10.4274/Jcrpe.1127.

/36.jpg)
/37_2.jpg)
/37_3.jpg)