Международный эндокринологический журнал Том 17, №2, 2021
Вернуться к номеру
Особливості перебігу діабетичної хвороби нирок у хворих на латентний автоімунний діабет дорослих
Авторы: I.O. Tsaryk, N.V. Pashkovska
Bukovinian State Medical University, Chernivtsi, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Актуальність. Латентний автоімунний діабет дорослих (LADA) є гетерогенним типом цукрового діабету (ЦД), що поєднує ознаки цукрового діабету 1-го (ЦД1) та 2-го типу (ЦД2). Дані щодо частоти і структури мікросудинних ускладнень при LADA малочисельні й доволі суперечливі, практично відсутня інформація про особливості їх перебігу, що вказує на необхідність проведення досліджень у цьому напрямку. Мета дослідження: з’ясувати особливості перебігу діабетичної хвороби нирок у хворих на латентний автоімунний діабет дорослих порівняно з класичними типами цукрового діабету. Матеріали та методи. Обстежено 112 хворих на ЦД із діабетичною хворобою нирок (ДХН). Пацієнтів розподілили на три групи: І — 54 особи з LADA, ІІ — 30 хворих на ЦД1, ІІІ — 28 пацієнтів із ЦД2. Особливості перебігу ДХН вивчали на підставі оцінки даних анамнезу, клінічного обстеження, значень швидкості клубочкової фільтрації (ШКФ), альбумінурії, відношення альбуміну до креатиніну в сечі. Результати. За даними анамнезу діагноз ДХН у пацієнтів із LADA встановлювався в середньому через 3 роки після маніфестації ЦД, при цьому на 4,5 року раніше, ніж при ЦД1, але на 1,3 року пізніше, ніж при ЦД2. Аналіз показників ШКФ показав, що при LADА найбільш часто (у 63 % пацієнтів) реєструвалась категорія G3 (G3а — у 46 %, G3b — у 17 % пацієнтів). Решта обстежених мали категорію G1 (7 %), G2 (24 %), і тільки в 6 % пацієнтів виявлено стадію G4. У пацієнтів усіх груп переважали категорії альбумінурії А1 та А2 (при LADА — по 43 % осіб у кожній категорії), водночас категорія A3 при LADA реєструвалась удвічі частіше, ніж при ЦД1. При LADA переважаючими фенотипами ДХН були неальбумінурійне порушення функції нирок (НАПН) (43 %) і альбумінурійний фенотип (АФ) (35 %), при ЦД1 — АФ (50 %) і НАПН (40 %), а при ЦД2 усі три фенотипи реєструвалися майже з однаковою частотою (АФ — у 32 %, НАПН — у 29 % пацієнтів, прогресуюче зниження функції нирок — у 39 % хворих). Висновки. Перебіг діабетичної хвороби нирок в осіб із латентним автоімунним діабетом дорослих відрізняється від такого при класичних типах цукрового діабету, що вказує на необхідність розробки специфічного алгоритму ведення цієї категорії пацієнтів.
Background. Latent autoimmune diabetes in adults (LADA) is a heterogeneous type of diabetes mellitus (DM) that combines symptoms of type 1 (T1DM) and type 2 diabetes mellitus (T2DM). Data about the frequency and structure of microvascular complications in LADA are small and quite contradictory, there is almost no information about the peculiarities of their course, which indicates the need for research in this area. Therefore, the purpose of the study was to determine the characteristics of diabetic kidney disease (DKD) in patients with latent autoimmune diabetes in adults compared with classical types of DM. Materials and methods. The study enrolled 112 patients with DM with DKD. Patients were divided into three groups: I — 54 people with LADA, II — 30 patients with T1DM, III — 28 patients with T2DM. Peculiarities of DKD course were studied based on the anamnesis data, clinical examination, glomerular filtration rate (GFR) values, albuminuria, albumin-creatinine ratio. Results. According to the anamnesis the diagnosis of DKD in patients with LADA was established on average 3 years after the manifestation of diabetes, which is 4.5 years earlier than in T1DM but 1.3 years later than in T2DM. The analysis of GFR stages showed that in LADA the category G3 was the most often (63 %) (G3a — in 46 %, G3b — in 17 %). The other patients had G1 stage (7 %), G2 stage (24 %), and only 6 % of patients had G4 stage. The albuminuria categories A1 and A2 predominated in patients of all groups (in LADA — 43 % of people in each category), while category A3 in LADA was registered twice as often as in T1DM. In LADA, the predominant phenotypes were non-albuminuric renal impairment (NARI) (43 %) and albuminuric phenotype (AP) (35 %), in T1DM — AP (50 %) and NARI (40 %), and in T2DM, all three phenotypes were registered with almost the same frequency (AP — in 32 %, NARI — in 29 %, progressive renal decline — in 39 %). Conclusions. The course of diabetic kidney disease in patients with latent autoimmune diabetes in adults differs from that in the classic types of diabetes, which indicates the need to develop a specific algorithm for this cohort of patients.
цукровий діабет; латентний автоімунний діабет дорослих; діабетична хвороба нирок
diabetes mellitus; latent autoimmune diabetes in adults; diabetic kidney disease
Introduction
Materials and methods
Results
/27.jpg)
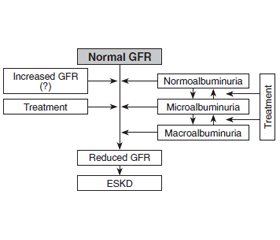
Discussion
/28_2.jpg)
Conclusions
- Pieralice S., Pozzilli P. Latent Autoimmune Diabetes in Adults: A Review on Clinical Implications and Management. Diabetes Metab. J. 2018. 42(6). 451-464. doi: 10.4093/dmj.2018.0190.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes — 2021. Diabetes Care. 2021. 44 (Suppl. 1). 15-33. doi: 10.2337/dc21-S002.
- Mishra R., Hodge K.M., Cousminer D.L., Leslie R.D., Grant S.F.A. A Global Perspective of Latent Autoimmune Diabetes in Adults. Trends Endocrinol. Metab. 2018. 29(9). 638-650. doi: 10.1016/j.tem.2018.07.001.
- Hawa M.I., Kolb H., Schloot N., Beyan H., Paschou S.A., Buzzetti R., Mauricio D. et al.; Action LADA consortium. Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: Action LADA 7. Diabetes Care. 2013. 36(4). 908-13. doi: 10.2337/dc12-0931.
- Buzzetti R., Tuomi T., Mauricio D. et al. Management of Latent Autoimmune Diabetes in Adults: A Consensus Statement from an International Expert Panel. Diabetes. 2020. 69(10). 2037-2047. e-mail: nvpashkovska@gmail.com; doi: 10.2337/dbi20-0017.
- Fu H., Liu S., Bastacky S.I., Wang X., Tian X.J., Zhou D. Diabetic kidney diseases revisited: A new perspective for a new era. Mol. Metab. 2019. 30. 250-263. doi: 10.1016/j.molmet.2019.10.005.
- Jiang G., Luk A.O.Y., Tam C.H.T., Xie F., Carstensen B., Lau E.S.H., Lim C.K.P. et al.; Hong Kong Diabetes Register TRS Study Group. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with type 2 diabetes. Kidney Int. 2019. 95(1). 178-187. doi: 10.1016/j.kint.2018.08.026.
- Maddaloni E., Lessan N., Al Tikriti A., Buzzetti R., Pozzilli P., Barakat M.T. Latent Autoimmune Diabetes in Adults in the United Arab Emirates: Clinical Features and Factors Related to Insulin-Requirement. PLoS One. 2015. 10(8). e0131837. doi: 10.1371/journal.pone.0131837.
- Carlsson S. Etiology and Pathogenesis of Latent Autoimmune Diabetes in Adults (LADA) Compared to Type 2 Diabetes. Front. Physiol. 2019. 10. 320. doi: 10.3389/fphys.2019.00320.
- Doshi S.M., Friedman A.N. Diagnosis and management of type 2 diabetic kidney disease. Clin. J. Am. Soc. Nephrol. 2017. 12. 1366-1373. doi: 10.2215/CJN.11111016.
- Pugliese G. Updating the natural history of diabetic nephropathy. Acta Diabetol. 2014. 51. 905-915. doi: 10.1007/s00592-014-0650-7.
- Yasui J., Kawasaki E., Tanaka S., Awata T., Ikegami H., Imagawa A., Uchigata Y. et al.; Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research. Clinical and Genetic Characteristics of Non-Insulin-Requiring Glutamic Acid Decarboxylase (GAD) Autoantibody-Positive Diabetes: A Nationwide Survey in Japan. PLoS One. 2016. 11(5). e0155643. doi: 10.1371/journal.pone.0155643.
- Shahbazian H., Rezaii I. Diabetic kidney disease; review of the current knowledge. J. Renal Inj. Prev. 2013. 2(2). 73-80. doi: 10.12861/jrip.2013.24.
- Kim M.K. Treatment of diabetic kidney disease: current and future targets. Korean J. Intern. Med. 2017. 32(4). 622-630. doi: 10.3904/kjim.2016.219.
- Levey A.S., Inker L.A., Coresh J. GFR estimation: from physiology to public health. Am. J. Kidney Dis. 2014. 63(5). 820-34. doi: 10.1053/j.ajkd.2013.12.006.
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021. 44 (Suppl. 1). 151-167. doi: 10.2337/dc21-S011.
- Fadiga L., Saraiva J., Catarino D., Frade J., Melo M., Paiva I. Adult-onset autoimmune diabetes: comparative analysis of classical and latent presentation. Diabetol. Metab. Syndr. 2020. 12(1). 107. doi: 10.1186/s13098-020-00616-1.
- Carlsson S. Environmental (Lifestyle) Risk Factors for LADA. Curr. Diabetes Rev. 2019. 15(3). 178-187. doi: 10.2174/1573399814666180716150253.
- Pugliese G., Penno G., Natali A., Barutta F., Di Paolo S., Reboldi G., Gesualdo L., De Nicola L.; Italian Diabetes Society and the Italian Society of Nephrology. Diabetic kidney disease: new clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function”. J. Nephrol. 2020. 33(1). 9-35. doi: 10.1007/s40620-019-00650-x.
- Ekinci E.I., Jerums G., Skene A., Crammer P., Power D., Cheong K.Y., Panagiotopoulos S. et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care. 2013. 36(11). 3620-6. doi: 10.2337/dc12-2572.
- Sulaiman M.K. Diabetic nephropathy: recent advances in pathophysiology and challenges in dietary management. Diabetol. Metab. Syndr. 2019. 11. 7. doi: 10.1186/s13098-019-0403-4.

/28.jpg)