Журнал «Боль. Суставы. Позвоночник» Том 13, №2, 2023
Вернуться к номеру
Остеопороз, артеріальна кальцифікація та сечокам’яна хвороба: сучасні методи боротьби зі старінням (огляд літератури)
Авторы: O.I. Nishkumay (1), Mike K.S. Chan (2), Yu.I. Nalapko (2), H.V. Mostbauer (1), O.O. Alekseenko (1), O.D. Nikitin (1), I.A. Kordubailo (1)
(1) — Bogomolets National Medical University, Kyiv, Ukraine
(2) — European Wellness Academy, Edenkoben, Germany
Рубрики: Ревматология, Травматология и ортопедия
Разделы: Справочник специалиста
Версия для печати
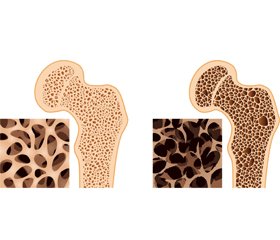
Актуальність. Проблема остеопорозу, як і серцево-судинних захворювань, залишається однією з головних у статистиці захворюваності та смертності через старіння населення в усьому світі. У сучасних публікаціях підкреслюють нову точку зору на зв’язок цієї статистики з механізмом «кальцієвого парадоксу». Ці процеси можуть мати загальні фактори ризику, що впливають на спільні механізми, коли ендотеліальні клітини різних органів перетворюються на бластоподібні та починають інтенсивно захоплювати кристали кальцію з відкладанням у судинній стінці та органах, наприклад нирках. Цей патологічний процес призводить до ламкості кісток і судин, нефрокальцинозу. Патогенез патологічної кальцифікації є складним, його механізм на сьогодні залишається не з’ясованим. Мета: проаналізувати поточні літературні дані про причини та молекулярні механізми ремоделювання кісток і кальцифікації судин відповідно до огляду літератури за останні 5 років. Матеріали та методи. Аналітичний огляд літературних даних (статті, резюме, метааналізи) було проведено з використанням інформаційного аналізу баз даних Medline (PubMed), Web of Science та Scopus, Google Scholar та Кокранівського центрального реєстру контрольованих досліджень (CENTRAL) за 2018–2023 рр. за ключовими словами «остеопороз», «атеросклероз», «кальцифікація судин», «стовбурові клітини», «екзосоми», «сечокам’яна хвороба». Результати. Аналіз літератури підкреслює численні патогенетичні механізми «кальцієвого парадоксу»: загальні фактори ризику (гіподинамія, дефіцит кальцію, вітаміну D та K2, паління), остеосаркопенію, імунне старіння й старіння стовбурових клітин. Висновки. Для вирішення проблеми «кальцієвого парадоксу» необхідна корекція багатьох механізмів: нормалізація добового вживання кальцію, вітамінів D та К2; профілактика причин, що викликають вторинний гіперпаратиреоз, падіння, остеосаркопенію, імунне старіння; вивчення можливостей застосування стовбурової терапії, мікроРНК та екзосом. Нове розуміння проблеми відкриває можливості для впливу на всі відомі ланки та впровадження нових методів лікування.
Background. The problem of osteoporosis as well as a cardiovascular disease remains one of the leading in the statistics of morbidity and mortality due to the aging of the population throughout the world. Recent publications accentuate the new viewpoint to an association of this statistic with the mechanism of the “calcium paradox”. These processes can have common risk factors when endothelial cells of different organs have been modified to osteoblasts-like bone cells and become intensive capture calcium crystals. This pathological process results in bone and vessel fragility and nephrocalcinosis. This pathogenesis is complex and common but not fully understood mechanisms. The purpose was to analyze the current literature data on reasons and molecular mechanisms of bone remodeling and vascular calcification according to a literature review over the past 5 years. Materials and methods. An analytical review of literature data was conducted using the information analysis of Medline (PubMed), Web of Science and Scopus databases, Google Scholar, and the Cochrane Central Register of Controlled Trials (CENTRAL) for 2018–2023 using the keywords “osteoporosis”, “atherosclerosis”, “vascular calcification”, “stem cells”, “exosomes”, “kidney stones diseases”. Results. The literature analysis underlines the multiplay pathogenetic mechanisms as common lifestyle risk factors (calcium and vitamin D, K2 deficiency smoking), as an osteosarcopenia, immunoaging, and stem cell senescence. Conclusions. In order to solve the problem prevention of the “calcium paradox”, it is necessary to access the correction of multiple mechanisms: calcium and vitamin D, K2 deficiency, reasons causing secondary hyperparathyroidism, falling, osteosarcopenia, immunoaging, and senescence of stem cells, microRNA and exosomes. A new understanding of the problem opens up opportunities for influencing all the known links and new perspectives of treatment.
артеріальна кальцифікація; остеопороз; остеопонтин; стовбурові клітини; екзосоми; сечокам’яна хвороба
arterial calcification; osteoporosis; osteopontin; stem cells; exosomes; kidney stone disease
Для ознакомления с полным содержанием статьи необходимо оформить подписку на журнал.
- Montero-Odasso M.M., Kamkar N., Pieruccini-Fa–ria F. et al. Evaluation of Clinical Practice Guidelines on Fall Prevention and Management for Older Adults: A Syste–matic Review. JAMA Netw. Open. 2021. 4(12). e2138911. doi: 10.1001/jamanetworkopen.2021.38911.
- Reid I.R. A broader strategy for osteoporosis interventions. Nat. Rev. Endocrinol. 2020. 16(6). 333-339. doi: 10.1038/s41574-020-0339-7.
- Adami G., Rahn E.J., Saag K.G. Glucocorticoid-induced osteoporosis: from clinical trials to clinical practice. Ther. Adv. Musculoskelet. Dis. 2019. 11. 1759720X19876468. doi: 10.1177/1759720X19876468.
- Ling Y., Xu F., Xia X. et al. Vitamin D supplementation reduces the risk of fall in the vitamin D deficient elderly: an updated meta-analysis. Clin. Nutr. 2021. 40(11). 5531-5537. doi: 10.1016/j.clnu.2021.09.031.
- Zanoli L., Lentini P., Briet M. et al. Arterial Stiffness in the Heart Disease of CKD. J. Am. Soc. Nephrol. 2019. 30(6). 918-928. doi: 10.1681/ASN.2019020117.
- Teo K.K., Rafiq T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can. J. Cardiol. 2021. 37(5). 733-743. doi: 10.1016/j.cjca.2021.02.009.
- García-Gómez M.C., Vilahur G. Osteoporosis and vascular calcification: a shared scenario. Osteoporosis y calcificación vascular: un escenario compartido. Clin. Investig. Arterioscler. 2020. 32(1). 33-42. doi: 10.1016/j.arteri.2019.03.008.
- Wang Z.X., Luo Z.W., Li F.X. et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat. Commun. 2022. 13(1). 1453. doi: 10.1038/s41467-022-29191-x.
- Sutton N.R., Malhotra R., St Hilaire C. et al. Mole–cular Mechanisms of Vascular Health: Insights From Vascular Aging and Calcification. Arterioscler. Thromb. Vasc. Biol. 2023. 43(1). 15-29. doi: 10.1161/ATVBAHA.122.317332.
- Komori T. Regulation of skeletal development by the Runx family of transcription factors. J. Cell. Biochem. 2005. 95(3). 445-453. doi: 10.1002/jcb.20420.
- Latic N., Erben R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020. 21(18). 6483. doi: 10.3390/ijms21186483.
- De la Guía-Galipienso F., Martínez-Ferran M., Vallecillo N., Lavie C.J., Sanchis-Gomar F., Pareja-Galeano H. Vitamin D and cardiovascular health. Clin. Nutr. 2021. 40(5). 2946-2957. doi: 10.1016/j.clnu.2020.12.025.
- Biffi A., Fernando F., Palermi S. et al. Cardiovascular disease prevention in the worksite: where are we? Int. J. Cardiol. 2022. 368. 104-107. doi: 10.1016/j.ijcard.2022.08.026.
- Guzon-Illescas O., Perez Fernandez E., Crespí Villarias N. et al. Mortality after osteoporotic hip fracture: incidence, trends, and associated factors. J. Orthop. Surg. Res. 2019. 14(1). 203. doi: 10.1186/s13018-019-1226-6.
- Katsanos S., Sioutis S., Reppas L. et al. What do hip fracture patients die from? Eur. J. Orthop. Surg. Traumatol. 2023. 33(4). 751-757. doi: 10.1007/s00590-022-03250-x.
- Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019. 393(10191). 2636-2646. doi: 10.1016/S0140-6736(19)31138-9.
- Migliorini F., Giorgino R., Hildebrand F. et al. Fragi–lity Fractures: Risk Factors and Management in the Elderly. Medicina (Kaunas). 2021. 57(10). 1119. doi: 10.3390/medicina57101119.
- Shoji T., Nakatani S,. Kabata D. et al. Comparative Effects of Etelcalcetide and Maxacalcitol on Serum Calcification Propensity in Secondary Hyperparathyroidism: A Randomized Clinical Trial. Clin. J. Am. Soc. Nephrol. 2021. 16(4). 599-612. doi: 10.2215/CJN.16601020.
- Trajanoska K., Rivadeneira F. The genetic architecture of osteoporosis and fracture risk. Bone. 2019. 126. 2-10. doi: 10.1016/j.bone.2019.04.005.
- Tada H., Fujino N., Hayashi K., Kawashiri M.A., Takamura M. Human genetics and its impact on cardiovascular disease. J. Cardiol. 2022. 79(2). 233-239. doi: 10.1016/j.jjcc.2021.09.005.
- Cosentino N., Campodonico J., Milazzo V. et al. Vitamin D and Cardiovascular Disease: Current Evidence and Future Perspectives. Nutrients. 2021. 13(10). 3603. doi: 10.3390/nu13103603.
- Reiss A.B., Miyawaki N., Moon J. et al. CKD, arterial calcification, atherosclerosis and bone health: inter-relationships and controversies. Atherosclerosis. 2018. 278. 49-59. doi: 10.1016/j.atherosclerosis.2018.08.046.
- Gracia-Marco L. Calcium, Vitamin D, and Health. Nutrients. 2020. 12(2). 416. doi: 10.3390/nu12020416.
- Michos E.D., Cainzos-Achirica M., Heravi A.S., Appel L.J. Vitamin D, Calcium Supplements, and Implications for Cardiovascular Health: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021. 77(4). 437-449. doi: 10.1016/j.jacc.2020.09.617.
- Bolland M.J., Avenell A., Baron J.A. et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010. 341. c3691. doi: 10.1136/bmj.c3691.
- Cheng C.H., Chen L.R., Chen K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022. 23(3). 1376. doi: 10.3390/ijms23031376.
- Cherukuri L., Kinninger A., Birudaraju D. et al. Coronary artery calcium and bone mineral density by serial CTA: does menopausal hormone therapy modify the association? Clin. Imaging. 2022. 90. 26-31. doi: 10.1016/j.clinimag.2022.06.023.
- Burte C., Lejeune H., Faix A. et al. Practical recommendations for the management of testosterone deficiency. Prog. Urol. 2021. 31(8–9). 458-476. doi: 10.1016/j.purol.2020.09.026 (in French).
- Baber R.J., Panay N., Fenton A.; IMS Writing Group. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016. 19(2). 109-150. doi: 10.3109/13697137.2015.1129166.
- Hodis H.N., Mack W.J. Menopausal Hormone Replacement Therapy and Reduction of All-Cause Mortality and Cardiovascular Disease: It Is About Time and Timing. Cancer J. 2022. 28(3). 208-223. doi: 10.1097/PPO.0000000000000591.
- Banjabi A.A., Kurunthachalam K., Kumosani T.A., Abulnaja K.O., Al-Malki A.L., Moselhy S.S. Serum heavy metals of passive smoker females and its correlation to bone biomarkers and risk of osteoporosis. Environ. Sci. Pollut. Res. Int. 2022. 29(5). 6943-6948. doi: 10.1007/s11356-021-16186-2.
- Bernabe-Ortiz A., Carrillo-Larco R.M. Second-hand smoking, hypertension and cardiovascular risk: findings from Peru. BMC Cardiovasc. Disord. 2021. 21(1). 576. doi: 10.1186/s12872-021-02410-x.
- Craig W.J., Mangels A.R., Fresán U. et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients. 2021. 13(11). 4144. doi: 10.3390/nu13114144.
- Le Daré B., Lagente V., Gicquel T. Ethanol and its metabolites: update on toxicity, benefits, and focus on immunomodulatory effects. Drug Metab. Rev. 2019. 51(4). 545-561. doi: 10.1080/03602532.2019.1679169.
- He F.J., Tan M., Ma Y., MacGregor G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020. 75(6). 632-647. doi: 10.1016/j.jacc.2019.11.055.
- Al-Hariri M., Aldhafery B. Association of Hypertension and Lipid Profile with Osteoporosis. Scientifica (Cairo). 2020. 2020. 7075815. doi: 10.1155/2020/7075815.
- Chen Y., Zhao X., Wu H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler. Thromb. Vasc. Biol. 2020. 40(5). 1078-1093. doi: 10.1161/ATVBAHA.120.313131.
- Kanis J.A., Norton N., Harvey N.C. et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021. 16(1). 82. doi: 10.1007/s11657-020-00871-9.
- Shevroja E., Cafarelli F.P., Guglielmi G., Hans D. DXA parameters, Trabecular Bone Score (TBS) and Bone Mineral Density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine. 2021. 74(1). 20-28. doi: 10.1007/s12020-021-02806-x.
- Jiang Y., Zhang P., Zhang X., Lv L., Zhou Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell. Prolif. 2021. 54(1). e12956. doi: 10.1111/cpr.12956.
- McDonald M.M., Khoo W.H., Ng P.Y. et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021. 184(5). 1330-1347.e13. doi: 10.1016/j.cell.2021.02.002.
- Yasuda H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021. 39(1). 2-11. doi: 10.1007/s00774-020-01175-1.
- Carrillo-López N., Martínez-Arias L., Fernández-Villabrille S. et al. Role of the RANK/RANKL/OPG and Wnt/β-Catenin Systems in CKD Bone and Cardiovascular Disorders. Calcif. Tissue Int. 2021. 108(4). 439-451. doi: 10.1007/s00223-020-00803-2.
- Dalle Carbonare L., Valenti M.T., Zanatta M., Donatelli L., Lo Cascio V. Circulating mesenchymal stem cells with abnormal osteogenic differentiation in patients with osteoporosis. Arthritis Rheum. 2009. 60(11). 3356-3365. doi: 10.1002/art.24884.
- Woodward H.J., Zhu D., Hadoke P.W.F., MacRae V.E. Regulatory Role of Sex Hormones in Cardiovascular Calcification. Int. J. Mol. Sci. 2021. 22(9). 4620. doi: 10.3390/ijms22094620.
- Boutouyrie P., Chowienczyk P., Humphrey J.D., Mitchell G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021. 128(7). 864-886. doi: 10.1161/CIRCRESAHA.121.318061.
- Farah B.Q., Cucato G.G., Andrade-Lima A. et al. Impact of hypertension on arterial stiffness and cardiac autonomic modulation in patients with peripheral artery disease: a cross-sectional study. Einstein (Sao Paulo). 2021. 19. eA06100. doi: 10.31744/einstein_journal/2021AO6100.
- Miyoshi T., Ito H. Arterial stiffness in health and di–sease: the role of cardio-ankle vascular index. J. Cardiol. 2021. 78(6). 493-501. doi: 10.1016/j.jjcc.2021.07.011.
- Mancusi C., Lembo M., Manzi M.V., Basile C., Fucile I., Morisco C. From Structural to Functional Hypertension Mediated Target Organ Damage — A Long Way to Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2022. 11(18). 5377. doi: 10.3390/jcm11185377.
- Cannata-Andía J.B., Carrillo-López N., Messina O.D. et al. Pathophysiology of Vascular Calcification and Bone Loss: Linked Disorders of Ageing? Nutrients. 2021. 13(11). 3835. doi: 10.3390/nu13113835.
- Tóth A., Balogh E., Jeney V. Regulation of Vascular Calcification by Reactive Oxygen Species. Antioxidants (Basel). 2020. 9(10). 963. doi: 10.3390/antiox9100963.
- Alesutan I., Henze L.A., Boehme B. et al. Periostin Augments Vascular Smooth Muscle Cell Calcification via β-Catenin Signaling. Biomolecules. 2022. 12(8). 1157. doi: 10.3390/biom12081157.
- Chellan B., Sutton N.R., Hofmann Bowman M.A. S100/RAGE-Mediated Inflammation and Modified Cholesterol Lipoproteins as Mediators of Osteoblastic Differentiation of Vascular Smooth Muscle Cells. Front. Cardiovasc. Med. 2018. 5. 163. doi: 10.3389/fcvm.2018.00163.
- Torzewski M. Enzymatically modified LDL, athe–rosclerosis and beyond: paving the way to acceptance. Front. Biosci. (Landmark Ed). 2018. 23(7). 1257-1271. doi: 10.2741/4642.
- Rashdan N.A., Sim A.M., Cui L. et al. Osteocalcin Regulates Arterial Calcification Via Altered Wnt Signaling and Glucose Metabolism. J. Bone Miner. Res. 2020. 35(2). 357-367. doi: 10.1002/jbmr.3888.
- Luzin V.I., Ivanova L.N., Nishkumaj O.I., Goroshko S.A. Osobennosti prochnosti kostej u belyh krys starche–skogo vozrasta, poluchavshih dietu s povyshennym soderzhaniem holesterina. Problemi osteologії. 2003. 7(3–4). 23-25 (in russian).
- Luo J., Yang Z., Ma Y. et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016. 22(5). 539-546. doi: 10.1038/nm.4076.
- Carrillo-López N., Martínez-Arias L., Alonso-Montes C. et al. The receptor activator of nuclear factor κΒ ligand receptor leucine-rich repeat-containing G-protein-coupled receptor 4 contributes to parathyroid hormone-induced vascular calcification. Nephrol. Dial. Transplant. 2021. 36(4). 618-631. doi: 10.1093/ndt/gfaa290.
- Almquist M., Isaksson E., Clyne N. The treatment of renal hyperparathyroidism. Endocr. Relat. Cancer. 2020. 27(1). R21-R34. doi: 10.1530/ERC-19-0284.
- Carrillo-López N., Panizo S., Alonso-Montes C. et al. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016. 90(1). 77-89. doi: 10.1016/j.kint.2016.01.024.
- Carrillo-López N., Panizo S., Alonso-Montes C. et al. High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol. Dial. Transplant. 2019. 34(6). 934-941. doi: 10.1093/ndt/gfy287.
- Tanaka S., Matsumoto T. Sclerostin: from bench to bedside. J. Bone Miner. Metab. 2021. 39(3). 332-340. doi: 10.1007/s00774-020-01176-0.
- Schunk S.J., Floege J., Fliser D., Speer T. WNT-β-catenin signalling — a versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021. 17(3). 172-184. doi: 10.1038/s41581-020-00343-w.
- Golledge J., Thanigaimani S. Role of Sclerostin in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2022. 42(7). e187-e202. doi: 10.1161/ATVBAHA.122.317635.
- Liu K.H., Fu J., Zhou N. et al. 1,25-Dihydroxyvitamin D3 Prevents Epithelial-Mesenchymal Transition of HMrSV5 Human Peritoneal Mesothelial Cells by Inhibiting Histone Deacetylase 3 (HDAC3) and Increasing Vitamin D Receptor (VDR) Expression Through the Wnt/β-Catenin Signaling Pathway. Med. Sci. Monit. 2019. 25. 5892-5902. doi: 10.12659/MSM.916313.
- Melguizo-Rodríguez L., Costela-Ruiz V.J., García-Recio E., De Luna-Bertos E., Ruiz C., Illescas-Montes R. Role of Vitamin D in the Metabolic Syndrome. Nutrients. 2021. 13(3). 830. doi: 10.3390/nu13030830.
- Chen T.K., Knicely D.H., Grams M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA. 2019. 322(13). 1294-1304. doi: 10.1001/jama.2019.14745.
- Islam A.K., Holt S., Reisch J., Nwariaku F., Antonelli J., Maalouf N.M. What Predicts Recurrent Kidney Stone after Parathyroidectomy in Patients with Primary Hyperparathyroidism? J. Am. Coll. Surg. 2020. 231(1). 74-82. doi: 10.1016/j.jamcollsurg.2020.04.015.
- Jia Q., Huang Z., Wang G. et al. Osteopontin: an important protein in the formation of kidney stones. Front. Pharmacol. 2022. 13. 1036423. doi: 10.3389/fphar.2022.1036423.
- Wang Z., Zhang Y., Zhang J., Deng Q., Liang H. Recent advances on the mechanisms of kidney stone formation (Review). Int. J. Mol. Med. 2021. 48(2). 149. doi: 10.3892/ijmm.2021.4982.
- Yang Y., Hong S., Wang Q., Wang S., Xun Y. Exosome-mediated crosstalk between epithelial cells amplifies the cell injury cascade in CaOx stone formation. J. Biol. Eng. 2023. 17(1). 16. doi: 10.1186/s13036-023-00324-0.
- Lok Z.S.Y., Lyle A.N. Osteopontin in Vascular Di–sease. Arterioscler. Thromb. Vasc. Biol. 2019. 39(4). 613-622. doi: 10.1161/ATVBAHA.118.311577.
- Bailey S., Sroga G.E., Hoac B. et al. The role of extracellular matrix phosphorylation on energy dissipation in bone. eLife. 2020. 9. e58184. doi: 10.7554/eLife.58184.
- Cao S., Li X., Feng T. et al. Hirudin promotes proliferation and osteogenic differentiation of –HBMSCs via activation of cyclic guanosine monophosphate (cGMP)/protein kinase-G (PKG) signaling pathway. Bioengineered. 2022. 13(3). 6061-6069. doi: 10.1080/21655979.2021.2008697.
- Stock M., Schett G. Vitamin K-Dependent Proteins in Skeletal Development and Disease. Int. J. Mol. Sci. 2021. 22. 9328. doi: 10.3390/ijms22179328.
- Halder M., Petsophonsakul P., Akbulut A.C. et al. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019. 20(4). 896. doi: 10.3390/ijms20040896.
- Jadhav N., Ajgaonkar S., Saha P. et al. Molecular Pathways and Roles for Vitamin K2–7 as a Health-Beneficial Nutraceutical: Challenges and Opportunities. Front. Pharmacol. 2022. 13. 896920. doi: 10.3389/fphar.2022.896920.
- Narvaez C.J., Bak M.J., Salman N., Welsh J. Vitamin K2 enhances the tumor suppressive effects of 1,25(OH)2D3 in triple negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 2023. 231. 106307. doi: 10.1016/j.jsbmb.2023.106307.
- Merra G., Dominici F., Gualtieri P. et al. Role of vitamin K2 in bone-vascular crosstalk. Int. J. Vitam. Nutr. Res. 2022. doi: 10.1024/0300-9831/a000761.
- Akbulut A.C., Pavlic A., Petsophonsakul P. et al. Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients. 2020. 12(6). 1852. doi: 10.3390/nu12061852.
- Mladěnka P., Macáková K., Kujovská Krčmová L. et al. Vitamin K — sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2022. 80(4). 677-698. doi: 10.1093/nutrit/nuab061.
- Fusaro M., Cianciolo G., Evenepoel P., Schur–gers L., Plebani M. Vitamin K in CKD Bone Disorders. Calcif. Tissue Int. 2021. 108(4). 476-485. doi: 10.1007/s00223-020-00792-2.
- Ma H., Zhang B.L., Liu B.Y. et al. Vitamin K2-Dependent GGCX and MGP Are Required for Homeostatic Calcium Regulation of Sperm Maturation. iScience. 2019. 14. 210-225. doi: 10.1016/j.isci.2019.03.030.
- Liang X., Ding Y., Zhang Y., Tse H.F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell. Transplant. 2014. 23(9). 1045-1059. doi: 10.3727/096368913X667709.
- Yang Z., Zhang W., Ren X., Tu C., Li Z. Exosomes: A Friend or Foe for Osteoporotic Fracture? Front. Endocrinol. (Lausanne). 2021. 12. 679914. doi: 10.3389/fendo.2021.679914.
- Yu H., Wang Z. Cardiomyocyte-Derived Exosomes: Biological Functions and Potential Therapeutic Implications. Front. Physiol. 2019. 10. 1049. doi: 10.3389/fphys.2019.01049.
- Qin Z., Liao R., Xiong Y. et al. A narrative review of exosomes in vascular calcification. Ann. Transl. Med. 2021. 9(7). 579. doi: 10.21037/atm-20-7355.
- Huang G., Zhao Q., Li W. et al. Exosomes: a new option for osteoporosis treatment. Medicine (Baltimore). 2022. 101(52). e32402. doi: 10.1097/MD.0000000000032402.
- Jiang W., Zhang Z., Li Y. et al. The Cell Origin and Role of Osteoclastogenesis and Osteoblastogenesis in Vascular Calcification. Front. Cardiovasc. Med. 2021. 8. 639740. doi: 10.3389/fcvm.2021.639740.
- Huang J., Xu Y., Wang Y. et al. Advances in the Study of Exosomes as Drug Delivery Systems for Bone-Related Diseases. Pharmaceutics. 2023. 15(1). 220. doi: 10.3390/pharmaceutics15010220.
- Pavlic A., Bahram Sangani N., Kerins J., Nicolaes G., Schurgers L., Reutelingsperger C. Vascular Smooth Muscle Cell Neutral Sphingomyelinase 2 in the Release of Exosomes and Vascular Calcification. Int. J. Mol. Sci. 2022. 23(16). 9178. doi: 10.3390/ijms23169178.
- Bommanavar S., Hosmani J., Togoo R.A. et al. Role of matrix vesicles and crystal ghosts in bio-mineralization. J. Bone Miner. Metab. 2020. 38(6). 759-764. doi: 10.1007/s00774-020-01125-x.
- Pavlic A., Poelman H., Wasilewski G. et al. Inhibition of Neutral Sphingomyelinase 2 by Novel Small Molecule Inhibitors Results in Decreased Release of Extracellular Ve–sicles by Vascular Smooth Muscle Cells and Attenuated Calcification. Int. J. Mol. Sci. 2023. 24(3). 2027. doi: 10.3390/ijms24032027.
- Ciceri P., Falleni M., Tosi D. et al. Therapeutic Effect of Iron Citrate in Blocking Calcium Deposition in High Pi-Calcified VSMC: Role of Autophagy and Apoptosis. Int. J. Mol. Sci. 2019. 20(23). 5925. doi: 10.3390/ijms20235925.
- Gardin C., Ferroni L., Leo S., Tremoli E., Zavan B. Platelet-Derived Exosomes in Atherosclerosis. Int. J. Mol. Sci. 2022. 23(20). 12546. doi: 10.3390/ijms232012546.
- Tkach M., Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016. 164(6). 1226-1232. doi: 10.1016/j.cell.2016.01.043.
- Dolz S., Górriz D., Tembl J.I. et al. Circulating MicroRNAs as Novel Biomarkers of Stenosis Progression in Asymptomatic Carotid Stenosis. Stroke. 2017. 48(1). 10-16. doi: 10.1161/STROKEAHA.116.013650.
- Qin Z., Li Y., Li J. et al. Exosomal STAT1 derived from high phosphorus-stimulated vascular endothelial cells induces vascular smooth muscle cell calcification via the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2022. 50(6). 139. doi: 10.3892/ijmm.2022.5195.
- Saengpanit D., Chattranukulchai P., Tumkosit M. et al. Effect of Sodium Thiosulfate on Arterial Stiffness in End-Stage Renal Disease Patients Undergoing Chronic Hemodialysis (Sodium Thiosulfate-Hemodialysis Study). A Randomized Controlled Trial. Nephron. 2018. 139(3). 219-227. doi: 10.1159/000488009.
- Raggi P., Bellasi A., Bushinsky D. et al. Slowing Progression of Cardiovascular Calcification With SNF472 in Patients on Hemodialysis: Results of a Randomized Phase 2b Study. Circulation. 2020. 141(9). 728-739. doi: 10.1161/CIRCULATIONAHA.119.044195.
- Lee B.C., Kang I., Yu K.R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021. 10(4). 711. doi: 10.3390/jcm10040711.
- Tan S.H.S., Wong J.R.Y., Sim S.J.Y. et al. Mesenchymal stem cell exosomes in bone regenerative strategies — a systematic review of preclinical studies. Mater. Today Bio. 2020. 7. 100067. doi: 10.1016/j.mtbio.2020.100067.
- Xeno: Liao Y.J., Tang P.C., Lin C.H., Chen L.R., Yang J.R. Porcine-induced pluripotent stem cell-derived osteoblast-like cells ameliorate trabecular bone mass of osteoporotic rats. Regen. Med. 2018. 13(6). 659-671. doi: 10.2217/rme-2018-0014.
- Xu F., Zhong J.Y., Lin X. et al. Melatonin alleviates vascular calcification and ageing through exosomal miR-204/miR-211 cluster in a paracrine manner. J. Pineal Res. 2020. 68(3). e12631. doi: 10.1111/jpi.12631.